Researchers from Cuba, China, and France have demonstrated the potential of coupling a viral antigen with the tetanus toxoid protein as a vaccination approach to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the agent that causes coronavirus disease 2019 (COVID-19).
Tetanus toxoid (TT) is a chemically inactivated version of the tetanus toxin produced by the bacteria Clostridium tetani. This chemically inactivated antigen can be used as a protein carrier in vaccines to induce potent immune responses in vivo.
The SARS-CoV-2 infection process is mediated by a surface structure called the spike protein. The receptor-binding (RBD) domain of this spike protein contains a receptor-binding motif (RBM) that mediates the interaction of the RBD with the host cell receptor angiotensin-converting enzyme 2 (ACE2).
This RBD-ACE2 interaction is the main target of the neutralizing antibodies that are generated following natural infection or vaccination.
Yury Valdes-Balbin from Finlay Vaccine Institute in Havana and colleagues have shown that a vaccine containing recombinant spike RBD conjugated to the tetanus toxin (TT) protein induces a potent immune response against SARS-CoV-2 in an animal model.
The team says the findings have paved the way for the evaluation of the RBD-TT conjugate in clinical trials.
“This paper demonstrates that subunit conjugate vaccines can be an alternative for COVID-19, paving the way for other viral conjugate vaccines based on the use of small viral proteins involved in the infection process,” write the researchers.
A pre-print version of the research paper is available on the bioRxiv* server, while the article undergoes peer review.
More about recombinant subunit vaccine platforms
The researchers say that the key advantages of recombinant subunit vaccine platforms are their safety and stability when stored at 2-8 °C and their suitability for large-scale production.
On the other hand, a disadvantage is a weak immunogenicity that would be expected for a small recombinant RBD protein (30 kDa), says the team. However, studies have demonstrated that administration of recombinant RBD with the adjuvant alum induced neutralizing immune responses in laboratory animals.
In addition to potentially weak immunogenicity, small recombinant RBD exposes camouflaged RBD to the immune system, which can fail to generate neutralizing antibodies.
Valdes-Balbin and colleagues hypothesized that once conjugated to TT, the orientation of RBD would improve the exposure of the RBM for immune recognition and increase the production of neutralizing antibodies.
What did the researchers do?
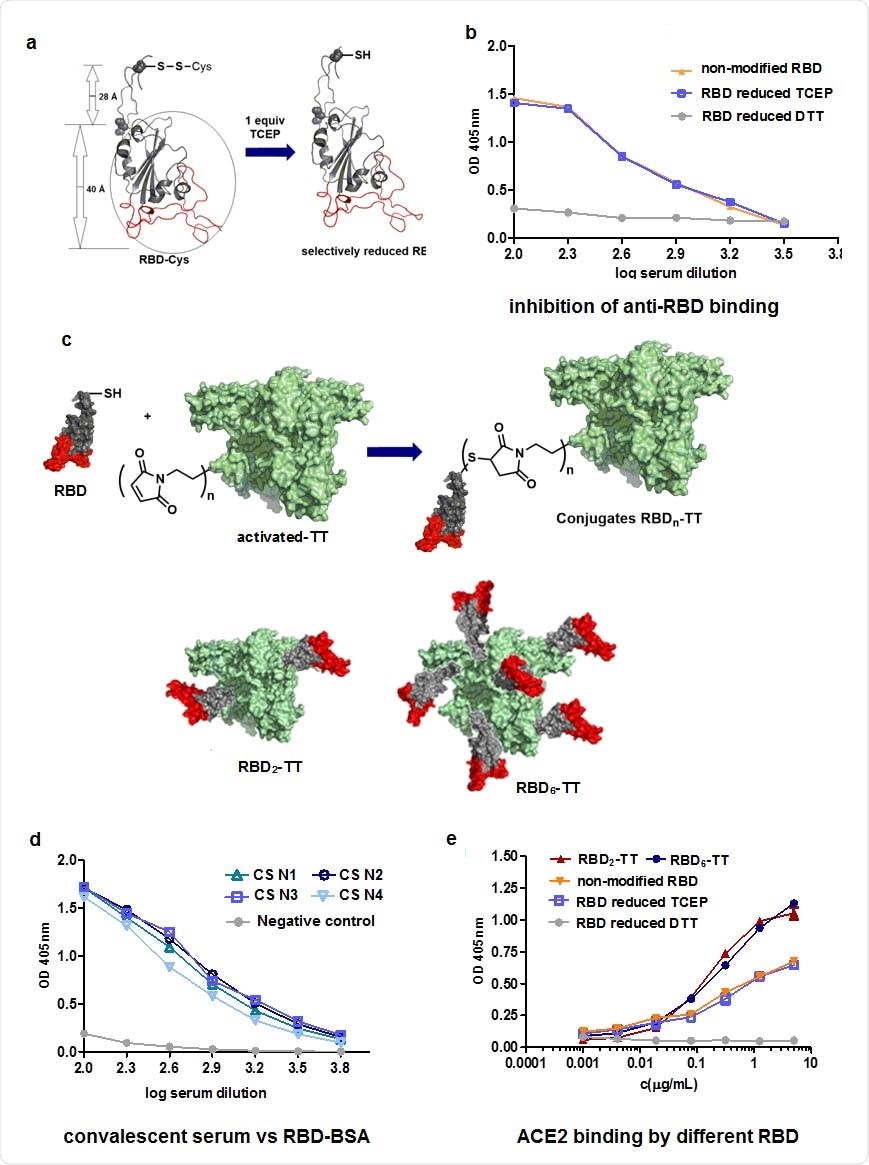
The team extended the 193-amino acid RBD sequence (residues Thr333 to Pro527) to include the spike residues 527 to 541 for the addition of Cys538, which is usually connected to Cys590. The RBD was also extended to include N-glycosylation sites at residues Asn331 and Asn343 and O-linked glycosylation sites at Thr323 and Ser325.
The resulting sequence yields a free Cys538 that can be used for chemical conjugation to the highly immunogenic TT carrier.
The researchers constructed RBD-TT conjugates bearing 2 or 6 mol of RBD per mol of TT (RBD2-TT and RBD6-TT).
“To our knowledge, chemically conjugated constructs and the immunogenic effect of conjugating viral proteins such as RBD to a protein carrier have not been assessed for SARS-CoV-2 or other coronaviruses,” says Valdes-Balbin and colleagues.
What did they find?
The analysis revealed that both the RBD2-TT and RBD6-TT conjugates showed slightly improved recognition of ACE2 than the original RBD. The team says this was probably due to improved exposure of the RBM.
Using an enzyme-linked immunosorbent assay (ELISA), the team compared the effects of immunizing BALB/c mice with RBD alone, RBD2– TT without alum, RBD6-TT with alum (RBD6-TT+alum) and RBD2-TT+alum.
Following one dose of the immunogens, RBD6-TT+alum induced the highest levels of anti-RBD antibodies.
After a second dose, all immunogens with alum induced higher anti-RBD immunoglobulin G (IgG) levels than those without alum.
“The high and homogeneous early response for RBD6-TT+alum could be an important attribute for a vaccine in pandemic times,” say the researchers.
When mice were immunized with two doses of RBD2-TT+alum, RBD6-TT+alum or RBD+alum, all of the antibody responses generated were effective at blocking the RBD–ACE2 interaction.
However, for RBD+alum, the neutralizing antibody titer was 232, whereas for RBD2-TT it was 1303, and for RBD6-TT it was 2568.
Furthermore, the neutralizing antibodies were mainly directed at the RBM.
The conjugates have entered clinical trials
“Here we show that macromolecular constructs with recombinant RBD conjugated to tetanus toxoid induce a potent immune response in laboratory animals,” write the researchers.
Valdes-Balbin and colleagues say that based on these findings, good manufacturing practices (GMP) batches of the RBD2-TT and RBD6-TT conjugates (with alum) entered clinical trials as final vaccine candidates.
Following the preliminary results of a phase I clinical trial initiated in October 2020, the vaccine based on RBD6-TT+alum was moved in December to a phase II clinical trial involving 910 participants.
“The encouraging results of the clinical trial will be published in due course,” says the team.
*Important Notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Valdes-Balbin Y, et al. SARS-CoV-2 RBD-Tetanus toxoid conjugate vaccine induces a strong neutralizing immunity in preclinical studies. bioRxiv, 2021. doi: https://doi.org/10.1101/2021.02.08.430146, https://www.biorxiv.org/content/10.1101/2021.02.08.430146v1
Posted in: Drug Trial News | Medical Research News | Disease/Infection News
Tags: ACE2, Amino Acid, Angiotensin, Angiotensin-Converting Enzyme 2, Animal Model, Antibodies, Antibody, Antigen, Assay, Bacteria, Cell, Clinical Trial, Clostridium, Conjugation, Coronavirus, Coronavirus Disease COVID-19, Enzyme, Glycosylation, Immune Response, Immune System, Immunoglobulin, in vivo, Laboratory, Manufacturing, Pandemic, Protein, Receptor, Research, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, T-Cell, Tetanus, Toxin, Vaccine

Written by
Sally Robertson
Sally has a Bachelor's Degree in Biomedical Sciences (B.Sc.). She is a specialist in reviewing and summarising the latest findings across all areas of medicine covered in major, high-impact, world-leading international medical journals, international press conferences and bulletins from governmental agencies and regulatory bodies. At News-Medical, Sally generates daily news features, life science articles and interview coverage.
Source: Read Full Article