 By Keynote ContributorProf. Ahmad Salehi, M.D, Ph.DClinical Professor with Stanford Medical School &
By Keynote ContributorProf. Ahmad Salehi, M.D, Ph.DClinical Professor with Stanford Medical School &Neurobiologist investigating the relationship between
Down syndrome and Alzheimer’s disease
By key contributors Jennifer Shinae Jennings, M.D. and Ahmad Salehi, M.D., Ph.D.
Every year, 5,300 new babies are born in the US with Down Syndrome (DS). These children face lifelong cognitive disability along with a number of life threatening medical issues including cardiovascular malformations and hematopoietic malignancies.
A young doctor viewing an ultrasound result to test for visible trisomy 21 signs. © Thomas Andreas / Shutterstock.com
Moreover, all adults with DS develop brain pathology similar to that of Alzheimer’s disease (AD). A number of new non-invasive prenatal testing methods allow us to detect DS earlier and more accurately than ever.
These tools offer an unprecedented opportunity for early treatment of brain abnormalities in DS. Currently, no therapy is available for cognitive dysfunction in DS and a recent clinical trial targeting a specific brain system in DS has not been very promising.
DS is caused by triplication of human chromosome 21 (HSA21). Extensive studies in individuals with triplication of fragments (rather than the entirety) of chromosome 21, along with mouse models with segmental trisomy, have led us to the following conclusion: The triplication of a number of HSA21 genes plays a more critical role in the occurrence of cognitive dysfunction in DS than others.
Chromosomes 21, Trisomy 21, Down Syndrome. © Jens Goepfert / Shutterstock.com
Numerous studies in humans have linked the triplication of specific regions of chromosome 21 to cognitive disability. These studies have been supported by the fact that deleting the extra copy of a number of triplicated genes in mouse models of DS can fix brain abnormalities.
Sequencing of HSA21 revealed the presence of 364-protein coding genes on this chromosome. Fortunately, HSA21 is the smallest and among the poorest chromosomes in terms of the number of functional genes.
Among the >300 genes, only a small number are expressed in the brain regions associated with learning and memory and have been linked to normal or abnormal functions in the nervous system. Normalizing the expression of a number of critical genes would be able to prevent a part of nervous system abnormalities in DS.
Possible gene targets
While triplication of a number of HSA21 genes could play a role in cognitive dysfunction in DS, two genes seem to play a more critical role: amyloid precursor protein (APP) gene and dual-specificity tyrosine phosphorylation kinase (DYRK1A) gene.
Amyloid precursor protein (APP)
DS individuals have triplication of a number of genes including the APP gene and protein levels are increased in the brains of people with DS. There are a number of studies that support the potential role of increased APP levels on cognitive disability in DS.
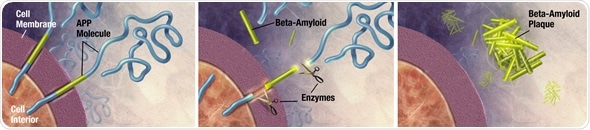
Enzymes act on the APP (Amyloid precursor protein) and cut it into fragments of protein, one of which is called beta-amyloid and its crucial in the formation of senile plaques in Alzheimer
The importance of APP is emphasized by adults with DS who do not have a triplication of APP (or a segment containing this gene), who do not necessarily develop severe brain atrophy and dementia of the AD type.
To support this, numerous mouse models have been generated for DS. Deleting the extra copy of the APP gene in mouse models leads to normalization of a number of brain abnormalities.
Further, a number of mutations in the APP gene have been shown to lead to the familial form of AD characterized by early onset (<65 years) and severe brain pathology.
A number of mutations in the APP gene lead to familial AD. Astonishingly, it has been revealed that in a number of cases, the mutations simply lead to the duplication of the APP gene. As the result, these individuals have three (instead of two) copies of APP and develop familial AD.
Dual-specificity tyrosine phosphorylation kinase (DYRK1A)
DYRK1A is an enzyme with an important role in neurodevelopment. This gene is also triplicated in DS, which increases the level of the enzyme in the brain.
Structure of protein DYRK1A.Based on PyMOL.
A number of studies support the importance of DYRK1A in cognitive dysfunction in DS with the following findings:
- DYRK1A mutations have been linked to cognitive disability in humans.
- DYRK1A inhibitors have shown to improve cognitive function in people with DS. For instance, EGCG is a member of the natural compounds found in in green tea leaves with the ability to inhibit DYRK1A and improve cognitive function in people with DS.
- Reducing the levels of DYRK1A in the hippocampus of mouse models of DS can improve learning and memory in these mice.
- Transgenic mice with increased levels of DYRK1A display deficits in the spatial learning.
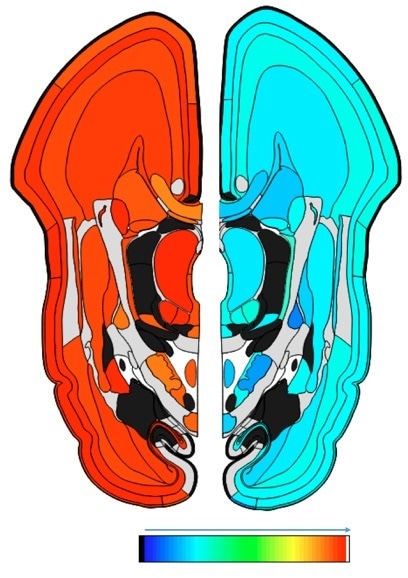
Both APP and DYRK1A genes are widely expressed in the developing human brain and play a significant role in a number of physiological functions of neurons and microglia. For these reasons, complete shutdown of either gene would impose significant developmental consequences in neuronal structure and function.
Fig. 1. The levels of gene expression for APP (right) and DYRK1A (left) in the human brain during the prenatal phase (15 weeks post conception). The colors depict the levels of gene expression ranging from the minimum (blue) to maximum (red). As shown both genes particularly, DYRK1A are expressed widely in the brain during early development.
© The Atlas of the Developing Human Brain, Allen Brain Atlas. http://www.brainspan.org/lcm/gene/348
For this reason, the best method to prevent cognitive dysfunction in the offspring would theoretically be achieved by partial reduction (by 50%) of either or both genes during the early stages of development.
Potential methods to target triplicated genes
X inactivation gene therapy
A number of new studies have put forward the idea of “turning off” the entire extra chromosome, essentially by employing the natural process that silences one of the X chromosomes contributed by all females.
Essentially, both X chromosomes in females contain an X inactivation gene (XIST) which produces a molecule, blocking other chromosomes from being expressed. The overexpression of the XIST gene could theoretically reduce the expression of most genes on HSA21.
Theoretically, this could lead to the normalization of the number of functional HSA21 from 3 to 2. The most important challenge is the specificity of the method targeting only triplicated genes in DS.
Antisense therapy
Following transcription, single strand RNA is formed from double strand DNA. Drugs with the ability to bind messenger RNA (mRNA) can prevent translation of RNA into protein or conversely prevent it from degrading.
A number of drugs have been developed on the basis on this strategy. One advantage that antisense drugs have in terms of therapeutic agents is their ability to cross the blood brain barrier.
Numerous studies have targeted mRNA molecules in cancer. Several drugs have already been approved by the FDA for the treatment of metabolic disorders and a few are in the pipeline seeking FDA approval.
Antisense molecules targeting APP mRNA have already been designed. The injection of antisense molecules targeting APP in mice with accelerated aging led to improvement of cognitive function in these mice.
DNA and RNA binding drugs
A number of DNA or RNA binding drugs could regulate the expression of a gene of interest. The strategy of using drugs targeting DNA is mostly popular in cancers. In fact, specific drugs can target DNA and destroy the cells bearing the genetic material of interest.
These compounds either directly damage DNA or damage enzymes involved in maintaining it. The lack of specificity and the potential for causing new malignancies can severely limit DNA binding proteins for neurodegenerative disorders.
Due to the presence of a number regulatory targets on mRNA, RNA binding drugs might be able to be effectively used in regulating the expression of individual genes. There are a number of RNA-binding drugs that have been developed that can reduce the expression of APP.
Pitfalls associated with in utero therapy
- Numerous regulatory and ethical issues come into play with genetic intervention in DS. Obviously, these issues must be addressed before moving forward.
- The issue of specificity is of great concern. Whether or not any of the methods mentioned above can truly target the genes of interest without altering the expression of a number of other genes is a question that must be answered.
- In almost all cases, normosomic mothers bear fetuses with trisomy 21. The challenge of limiting the effects of reducing the genes of interest in the fetus without affecting mothers is a complex issue that must be addressed.
- Causing malformations and toxicity in the fetus is of great concern when drugs are administered during pregnancy.
Conclusions
While a number of clinical studies have indicated that it is possible to partially improve cognitive function in children and adults with DS, most current therapeutic strategies are symptomatic and would only fix limited aspects of DS.
A fundamental treatment for learning disability in DS would constitute reducing the expression of genes like APP and DYRK1A, with important functional implications on cognitive function. There are examples of studies in animal models, in which the treatment of pregnant mice carrying fetuses with trisomy of DS-related genes has been able to significantly improve cognitive function in the adult offspring.
The availability of non-invasive methods for prenatal diagnosis of DS as early as 10 weeks, provides us with ~30-week window of opportunity for the treatment and regulation of the expression of triplicated genes.
We are hoping that the overview presented here will help create alternative paths for fundamental treatment of intellectual disability in DS. The complex ethical and scientific issues of gene therapy in DS have yet to be fully resolved.
Considering the enormous advancement in developing new diagnostic and genetic manipulation methods during the last several years, solving these issues seems more achievable than before. Once completed, it would provide families of children with DS the option to take these preventative measures.
Further Reading
- All Down Syndrome Content
- Down Syndrome – What is Down Syndrome?
- Down Syndrome Symptoms
- Down Syndrome Complications
- Down Syndrome Screening
About Jennifer Shinae Jennings and Ahmad Salehi
Jennifer Jennings is a neurosurgeon and a Stanford alumna. She is currently working as a Research Fellow at the VA Palo Alto Health Care System in Palo Alto, CA, focusing on traumatic brain injury and neurodegenerative diseases.
 Ahmad Salehi is a Clinical Professor affiliated with Stanford Medical School and a neurobiologist working on the relationship between Down syndrome and Alzheimer’s disease. He has been involved in identifying neuronal networks that undergo significant degeneration in mouse models of Down syndrome and Alzheimer’s disease.
Ahmad Salehi is a Clinical Professor affiliated with Stanford Medical School and a neurobiologist working on the relationship between Down syndrome and Alzheimer’s disease. He has been involved in identifying neuronal networks that undergo significant degeneration in mouse models of Down syndrome and Alzheimer’s disease.
Recently, Salehi and colleagues, edited a book on similarities between Down syndrome and Alzheimer’s disease. Furthermore, in a double-blind placebo-controlled clinical trial, he is currently testing whether an already FDA-approved drug can improve cognitive function in individuals with mild to moderate dementia of Alzheimer type.
Ahmad Salehi, M.D. Ph.D.
Clinical Professor
Department of Psychiatry & Behavioral Sciences
Stanford Medical School
VA Palo Alto Health Care System
3801 Miranda Ave, Y 151, Palo Alto, CA 94304
www.ahmadsalehi.info
Disclaimer: This article has not been subjected to peer review and is presented as the personal views of a qualified expert in the subject in accordance with the general terms and condition of use of the News-Medical.Net website.
Last Updated: Jun 25, 2019
Source: Read Full Article