An analysis of global usage patterns of a common hypertension drug following a major recall demonstrates the worldwide impact of contamination at a single manufacturing facility.
The research, led by researchers at the University of Toronto and published in BMJ Open, highlights the vulnerability of the global drug supply chain and the impact of drug recalls on access to safe medications, particularly in lower-income countries.
“There is very little research that has described global drug shortages or monitored how a single event can ripple around the world,” says Mina Tadrous, assistant professor in U of T’s Leslie Dan Faculty of Pharmacy. “This research demonstrates that drug shortages and recalls are global and impact use around the world. And there’s evidence that they don’t affect every country in the same way.”
Valsartan, where to buy cheap levirta dapoxetine au overnight shipping which is sold under several brand names, is a drug taken daily to treat high blood pressure and heart failure. In 2018, the European Medicines Agency detected that its supply of valsartan was contaminated by a potential carcinogen. The contamination was traced back to a single supplier of the active pharmaceutical ingredient (API), which had been manufactured in China and distributed to various manufacturers. Following the initial recall in Europe, other countries and manufacturers around the world voluntarily recalled batches of valsartan over the following few months.
Yuna Choi, a fourth-year student in the doctor of pharmacy (PharmD) program, worked with Tadrous to analyze data on the monthly purchases of antihypertensive medications in 83 countries in the period immediately before and after the recall. They aimed to determine whether purchase patterns of valsartan and other classes of hypertension drugs changed following the recall in countries of different economic status.
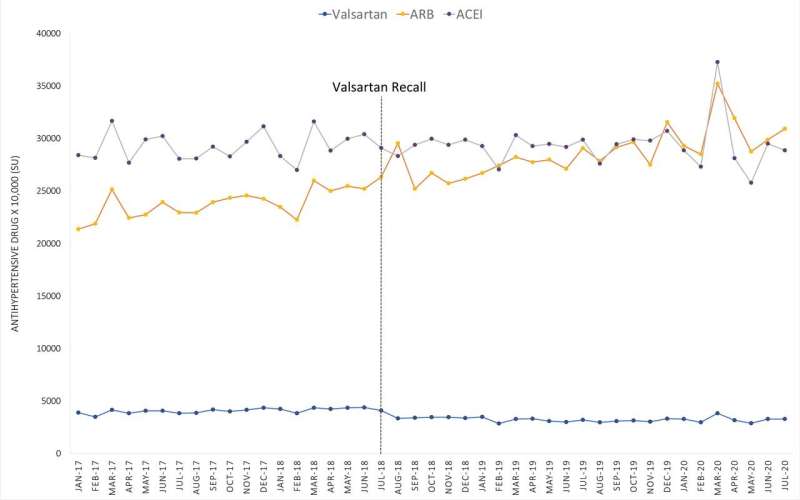
Their analysis showed valsartan use globally dropped significantly after the recall, but not every country changed their use in the same way. In higher-income countries, valsartan use dropped overall after the recall. Most lower-income countries, by contrast, showed similar or an overall increase in valsartan use.
Because the data in the analysis contained information about the overall consumption of valsartan in each country and did not distinguish between manufacturers or the source of API, the analysis could not offer any explanation for the differences in valsartan purchases between countries, Choi explains.
However, the finding does raise important questions about how each country managed the recall and, the study notes, “concerns of potential distribution of contaminated medications from developed countries to developing countries.”
“With globalization of the drug supply chain, disruptions can lead to global drug shortages with detrimental impacts on patient health outcomes and cause burden to the health care system,” Choi says. “Yet, this study showed countries of different economic statuses are impacted by the recall to a differing extent.”
The analysis also provides lessons for what countries can do to protect themselves from future recalls.
“We also saw some countries did not change their purchasing patterns, perhaps by having a diverse supply chain or being protected in some other way. There might be an opportunity to learn from other countries that weren’t as affected by the recall,” Tadrous says. “Every country seems to be tackling this problem on their own, and there needs to be a concerted effort to work together to protect supply chains.”
The global drop in valsartan use following the recall also highlights the vulnerability of the drug supply chain to shortages from even just one local problem and gaps in accessing safe medications.
“Contamination in one local facility led to a global impact that affected millions of patients who take this medication every day,” Choi says. “If pharmaceutical manufacturers are relying on the same source of API as other manufacturers, how can we claim that we have options and good backup supplies in the case of a recall?
“These shortages and recalls can have significant consequences on clinical outcomes and health system costs, but they can be prevented through policy and strategy to address the underlying causes and mitigate their effects globally.”
Pandemic highlighted drug shortages and supply-chain issues
Choi has been interested in examining the impact of pharmacy policy and regulations since starting the PharmD program. Undertaking the research project with Tadrous allowed her to explore a different side of the profession. As she nears the end of the program, she says she will be pursuing a master’s degree in pharmaceutical science at the Leslie Dan Faculty of Pharmacy to continue examining issues related to access to safe medications.
She hopes that the research on the valsartan recall will raise more awareness of the disproportionate impact of drug shortages on lower-income countries and the need for a global effort to improve access to medications—an issue that became more common during the COVID-19 pandemic.
“Drug shortages are not a new problem, but COVID-19 has certainly shed light on these drug shortages,” Choi says. “It’s important that there is a global effort to ensure that patients worldwide have access to medication—and that countries work together to ensure that medication supplies are safe.”
More information:
Yuna Choi et al, Effects of the July 2018 worldwide valsartan recall and shortage on global trends in antihypertensive medication use: a time-series analysis in 83 countries, BMJ Open (2023). DOI: 10.1136/bmjopen-2022-068233
Journal information:
BMJ Open
Source: Read Full Article