Researchers at DZNE and Charité–Universitätsmedizin Berlin have pioneered a novel treatment for the most common autoimmune encephalitis. By reprogramming white blood cells to target and eliminate disease-causing cells, the approach offers a new level of precision and efficiency. The technique has proven successful in laboratory studies, clinical trials in humans are already being planned.
NMDA receptor encephalitis is the most common form of antibody-caused brain disease, in which antibodies suddenly attack the brain, thus turning against the patient’s own body. “In pre-clinical testing, we have succeeded in switching off selectively the cells in which these misdirected antibodies are formed,” says Professor Harald Prüss, who conducts research at DZNE’s Berlin site and at Charité–Universitätsmedizin Berlin.
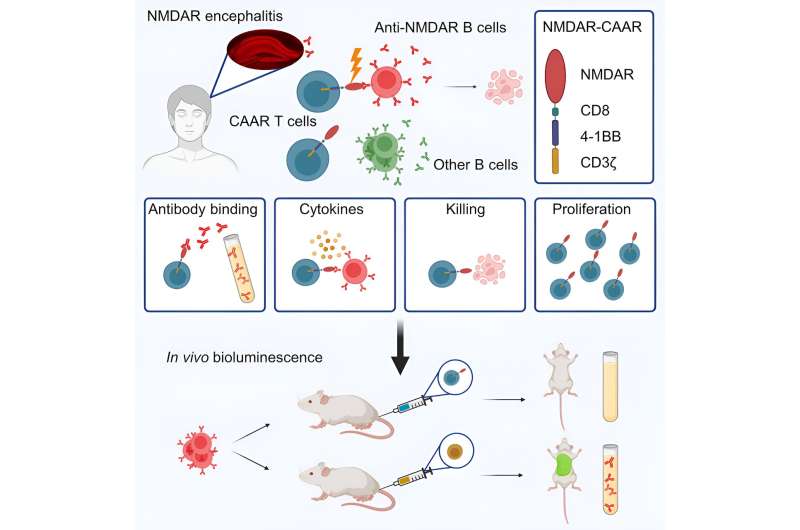
The team has engineered specialized CAAR T cells, designed for injection into patients. These “chimeric autoantibody receptor T cells” are programmed to identify and eliminate, cephalexin may cause itching with high precision, the cells responsible for producing harmful antibodies. In mouse models, this innovative approach has already demonstrated its accuracy. The findings have been published by Prüss and colleagues in the journal Cell.
New treatment method
About 200 to 300 people are estimated to develop NMDA receptor encephalitis in Germany every year—with severe symptoms. They experience memory impairment, epileptic seizures, impaired consciousness, and psychosis. Severe cases may even require treatment in an intensive care unit. The new procedure would be an enormous improvement over current therapy.
“Instead of suppressing the entire immune system and eliminating not only the misdirected antibodies, but also the more than 99% of beneficial antibodies, as we have done in the past, we set out to find a targeted approach.” That has now been achieved. “You can think of it like a surgical procedure, where the scalpel cuts out only what is harmful and the healthy tissue is left untouched,” Prüss says.
The disease, against which the therapy is directed, is insidious. The patient’s own immune system becomes the enemy, attacking the body instead of protecting it. While antibodies designed to eliminate viruses or bacteria are abundant in human blood, problems arise when some of these antibodies mistakenly target the body’s own tissues. If they cross the so-called blood-brain barrier, they can attack the brain. It is known by now that there is a whole range of such “autoimmune encephalopathies,” each triggered by different antibodies.
Reprogrammed cells
For their new, targeted therapy, the researchers had to develop an elaborate procedure. “We use human T cells, which we can obtain from patients’ blood, and modify them by adding a coupling molecule,” explains Dr. Momsen Reincke, one of the study’s first authors, who also researches at DZNE and Charité. This genetic reprogramming turns the T cells—a special type of white blood cells—into what is known as CAAR T cells.
Once reintroduced into the body, these CAAR T cells now specifically attack those body cells that produce the misdirected antibodies. “The surface of these cells is shaped in such a way that the CAAR T cells precisely dock onto them and kill them.” Importantly, cells producing other antibodies and therefore have a different surface, remain untouched.
The term “chimera” behind the acronym CAAR refers to the principle of artificially building a molecule from different components. A treatment that follows a similar pattern already exists in cancer therapy. The Berlin researchers are the first to successfully apply this concept to an autoimmune disease of the brain.
Prospects for cure?
In a next step, the scientists aim to test the new therapy in a human clinical trial. This could start in one to two years, according to current estimates. “In the beginning, we will take a blood sample from every affected person in order to obtain T cells from them individually,” says Prüss. “Given the rapid developments in the field of cell therapies, however, the next step could presumably be to use cells with which treatment no longer has to be patient-specific. That would be far less costly.”
The hope of the DZNE researchers is that a single application of the reprogrammed cells could definitively cure this type of autoimmune encephalitis, because, according to current knowledge, once killed, the cells that generate the problematic antibodies usually do not reproduce. Moreover, the method could be adapted to work in other autoimmune encephalopathies and not only in the NMDA receptor variant.
More information:
S. Momsen Reincke et al, Chimeric autoantibody receptor T cells deplete NMDA receptor-specific B cells, Cell (2023). DOI: 10.1016/j.cell.2023.10.001
Journal information:
Cell
Source: Read Full Article