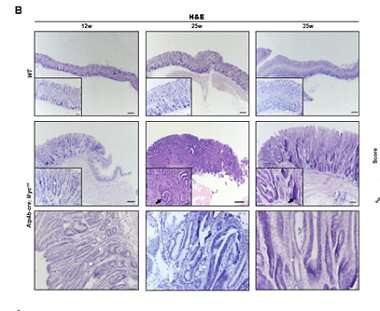
 generic topamax next day no prescription 50μm) of the fundic stomach mucosa from Atp4b-cre; MycOE and WT mice at 12, 25, and 35 weeks of age. The second row of HE pictures from Atp4b-cre; MycOEshowed IM. White asterisk indicated atrophy with loss of parietal cells and chief cells. Black asterisk indicated hyperplasia. White arrows indicated the presence of goblet cells. Black arrows indicated dysplasia. Hollow arrow indicated inflammation. Histologic scores of fundus from Atp4b-cre; MycOE mice (n ≥ 3) were evaluated and showed at right. A score of 4 denotes the highest pathologic severity and a score of 0 denotes the normal condition. Credit: Liu J, Feng W, Liu M, Rao H, Li X, Teng Y, Yang X, Xu J, Gao W, Li L. Stomach-specific c-Myc overexpression drives gastric adenoma in mice through AKT/mammalian target of rapamycin signaling. Bosn J of Basic Med Sci. 2020, Nov.19″ width=”380″ height=”311″>
generic topamax next day no prescription 50μm) of the fundic stomach mucosa from Atp4b-cre; MycOE and WT mice at 12, 25, and 35 weeks of age. The second row of HE pictures from Atp4b-cre; MycOEshowed IM. White asterisk indicated atrophy with loss of parietal cells and chief cells. Black asterisk indicated hyperplasia. White arrows indicated the presence of goblet cells. Black arrows indicated dysplasia. Hollow arrow indicated inflammation. Histologic scores of fundus from Atp4b-cre; MycOE mice (n ≥ 3) were evaluated and showed at right. A score of 4 denotes the highest pathologic severity and a score of 0 denotes the normal condition. Credit: Liu J, Feng W, Liu M, Rao H, Li X, Teng Y, Yang X, Xu J, Gao W, Li L. Stomach-specific c-Myc overexpression drives gastric adenoma in mice through AKT/mammalian target of rapamycin signaling. Bosn J of Basic Med Sci. 2020, Nov.19″ width=”380″ height=”311″>
Gastric cancer is one of the most common malignant cancers worldwide. Since the stage of early gastric cancer usually lasts for a long time and lacks obvious symptoms, the diagnosis of early gastric cancer is very difficult. Therefore, gastric cancer patients typically have a poor prognosis and low survival rate. During the progression of gastric cancer, genetic factors play an indispensable role. c-Myc, an oncogene, is abnormally highly expressed in a variety of tumors, including gastric cancer. For example, the Hi-Myc mouse model with high expression of c-Myc has been widely used in prostate cancer research. However, there is still no gastric cancer mouse model with increased expression of c-Myc, and it is unclear how c-Myc promotes gastric cancer in the early stage.
In the study published in the Bosnian Journal of Basic Medical Sciences, the authors successfully generated a stomach-specific c-Myc transgenic mouse model. They found that overexpression of c-Myc in Atp4b+ gastric parietal cells could induce gastric adenoma in mice. Mechanistically, c-Myc promoted tumorigenesis via the AKT/mTOR pathway. Furthermore, AKT inhibitor (MK-2206) or mTOR inhibitor (Rapamycin) repressed the proliferation of c-Myc overexpressing gastric cancer cells and the gastric tumorigenesis in c-Myc transgenic mice.
In general, the authors of the study first established a stomach-specific c-Myc transgenic mouse model, which provided a brand-new animal model for subsequent gastric cancer research. Also, their findings highlight that gastric tumorigenesis induced by c-Myc overexpression is through activation of the AKT/mTOR pathway.
Source: Read Full Article