A study by chemists at the University of Chicago has uncovered a new key step in the process that HIV uses to replicate itself.
The study, published Jan. 6 in Science Advances, used computer modeling to focus on how HIV forms a capsule that carries its genetic material—in particular, the role of a particular ion known as IP6. Scientists had previously suspected IP6 has an important function but didn’t know exactly how it worked.
The theory may explain aspects of the success of a promising new drug, as well as point the way to other treatments.
Replicating replication
We have known for many years that HIV is the dangerous virus behind AIDS, but we know much less about exactly how it behaves when it enters the body. Unraveling the complex series of biochemical events can show us where and how the virus can be fought.
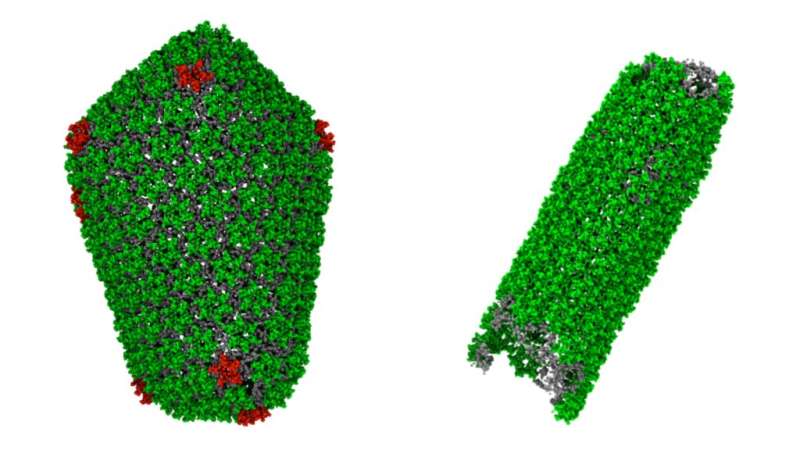
HIV carries its genetic material inside a little capsule known as a capsid.
“This capsid has been a target for HIV researchers for years,” said Gregory Voth, the Haig P. Papazian Distinguished Service Professor of Chemistry at the University of Chicago and the senior author on the paper. “The thought is that if we could stop the capsids from forming, or cause them to malform, we could prevent HIV from being infectious and replicating.”
The capsid is made up of about a thousand proteins, formed into either six-sided shapes or five-sided shapes, that assemble like bricks to make an enclosed structure. Scientists knew that an ion known as IP6 is also important in the process, but not precisely why.
This was a perfect question for Prof. Voth’s research group which specializes in using computer simulations to understand the behavior of complex molecules.
Ph.D. student Manish Gupta, the first author on the paper, built a model of the ingredients for the HIV capsid. Then the team ran multiple simulations with slightly different variations to find out how the capsid assembled, and which parts were critical.
From their previous studies, the team knew that the IP6 ion favors sitting in the middle of the five-sided structures. But their new simulations revealed that a few IP6 ions must bind into these five-sided structures to stabilize them very early during the capsid assembly in order to eventually close up.
“As the capsid starts to assemble, these five-sided structures create a region of high curvature, which the capsid needs to eventually close off the end,” explained Voth. “If they don’t, the structure starts to form a tube that is open at both ends, and it cannot close.”
If the process results in tubes instead of enclosed shapes, the genetic material cannot be enclosed and cannot be carried to its next target. The virus fails to replicate.
Though IP6 is tiny and only affects a dozen out of the many hundreds of molecules involved, it plays an outsize role in the process.
“The IP6 needs to be involved right at the beginning, too. It’s kind of like a drag race; if you don’t get the right speed off the starting line, you’re finished,” Voth said.
Critical findings
The result may explain the promise of a drug currently in human trials: lenacapavir. Lenacapavir “competes” with IP6, stabilizing the six-sided structures over the five-sided ones. This tilts the process to favor open-ended tube shapes instead of enclosed capsids.
“This is a really exciting result. Anything that is critical to the virus assembly can be a drug target, so we are always looking for these critical stages,” Voth explained.
Voth also said the team is interested in finding whether this process is more general to viruses beyond HIV. “Maybe these ‘minor’ participants are more important than we thought,” he said.
These are incredibly complex simulations, involving a million or more individual atoms; they must be performed on a supercomputer. These were run at the Texas Advanced Computing Center (TACC) at the University of Texas at Austin on the Frontera supercomputer.
More information:
Manish Gupta et al, Critical mechanistic features of HIV-1 viral capsid assembly, Science Advances (2023). DOI: 10.1126/sciadv.add7434
Journal information:
Science Advances
Source: Read Full Article