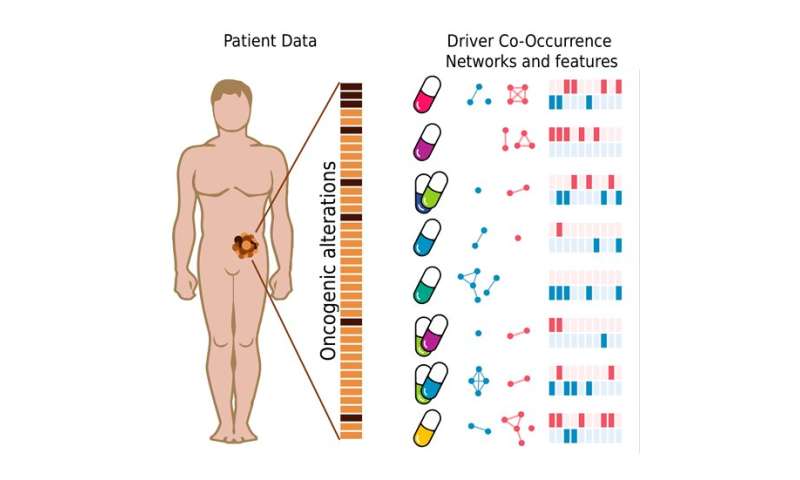
Cancer driver genes are those with mutations that are essential for tumor development and spread. Led by ICREA researcher Patrick Aloy, scientists from the Structural Bioinformatics and Network Biology (SBNB) Laboratory at IRB Barcelona have developed a computational pipeline that predicts tumor response to different cancer treatments. This system is based on the identification of complex response markers derived from the patterns of co-occurrence between cancer driver genes carrying mutations. It has been tested experimentally and with data from breast cancer patients and has achieved 66% accuracy in the prediction of these responses.
Given their key role in tumor development, cancer driver genes have been widely studied in recent decades. Knowledge of which of these genes are affected in a specific tumor can help to identify the most appropriate therapeutic strategy for that patient, in an approach known as precision medicine. For the first time, researchers from the SBNB Lab propose the co-occurrence (or lack thereof) of alterations in two or more cancer driver genes as a key factor in predicting the response to a certain treatment.
“The sum of two or more cancer driver genes affected by mutations leads to the formation of a complex network of biomarkers, alters the molecular profile of the tumor and affects its response to treatments,” says Aloy. “Through this work, we see that studying cancer driver genes as a whole, analyzing the different combinations, can bring about a great advance towards precision medicine,” he adds.
From bioinformatics to experimental and clinical analysis
Although there are a lot of data on cancer genomes, less information is available on the outcome of therapeutic interventions in patients. The researchers started from a public database that collects information on the effect of multiple treatments on the growth of human tumors that have been implanted in a mouse model. Based on these data, they selected 53 treatments (or combinations of treatments) and compared the molecular profiles of tumors that responded to each treatment and those that did not.
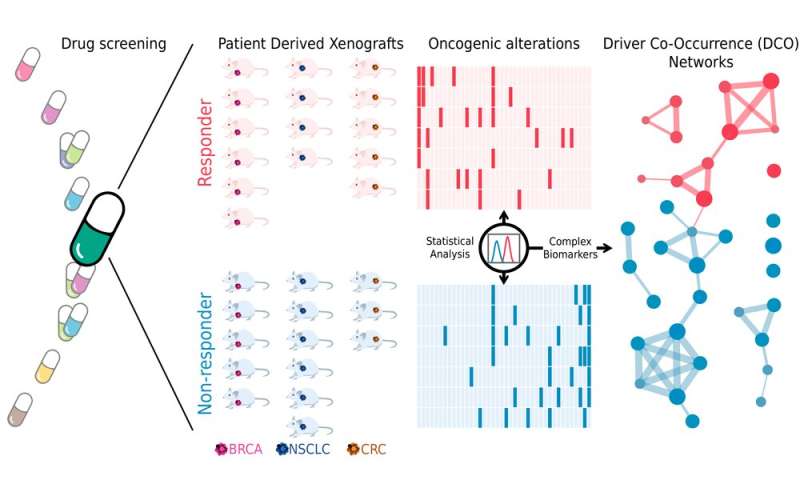
“After developing our computational model, we validated it experimentally in human tumors implanted in mice,” says Lídia Mateo, postdoctoral researcher at the SBNB Lab and first author of the study. “We were able to predict the outcome of the therapy in 12 of the 14 case studies, well above the power of approved biomarkers to predict drug response,” she adds. The researchers also validated the algorithm with treatment response data from patients with breast cancer.
Source: Read Full Article