During the early stages of the COVID-19 pandemic, where there was an absence of treatment or vaccine against severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2), several studies conducted worldwide screened for substances that could inhibit SARS-CoV-2 infection, replication, and propagation from marine bioactive compounds that included polyphenols, polysaccharides, and carotenoids.
Polyphenols obtained from brown algae, also known as phlorotannins, were predicted to be an inhibitor of SARS-CoV-2 in silico. Siphonaxanthin, which is a marine carotenoid obtained from Codium fragile, could inhibit the entry of SARS-CoV-2 pseudovirus into the cell in vitro.
Recent studies have indicated that iota‐carrageenan can inhibit SARS-CoV-2 replication in several cell lines. It has also been reported to inhibit the replication of SARS-CoV-2 variants, alpha, beta, gamma, and delta. Additionally, an iota‐carrageenan nasal spray was also identified that reduced the risk of SARS-CoV-2 infection in healthcare workers who managed patients with COVID-19 by 79.8 percent.
Furthermore, several marine sulfated polysaccharides were observed to inhibit SARS-CoV-2 entry by interfering with the interaction between the ACE2 receptor of the host cell and the SARS-CoV-2 spike protein in vitro. A previous study showed that crude polysaccharides (CPs) from seaweed and abalone viscera could effectively inhibit SARS-CoV-2 pseudovirus entry.
A new study published in Marine Drugs evaluated the inhibitory effect of three polysaccharides, crude polysaccharide from Hizikia fusiforme (CPHF), crude polysaccharide from Sargassum horneri (CPSH), and crude polysaccharide from abalone viscera (CPAV) on the propagation of SARS-CoV-2 in vitro.
 Study: Evaluation of Antiviral Effect against SARS-CoV-2 Propagation by Crude Polysaccharides from Seaweed and Abalone Viscera In Vitro. Image Credit: mnimage / Shutterstock
Study: Evaluation of Antiviral Effect against SARS-CoV-2 Propagation by Crude Polysaccharides from Seaweed and Abalone Viscera In Vitro. Image Credit: mnimage / Shutterstock
About the study
The study involved cytotoxicity evaluation of CPHF, CPSH, and CPAV followed by plaque reduction assay using different concentrations of CPHF, CPSH, and CPAV. The 50 percent tissue culture infectious dose (TCID50) was determined based on whether a cytopathic effect occurred or not.
An immunofluorescence assay was performed to assess the propagation of SARS-CoV-2. Western blotting was carried out to examine the expression of the viral spike (S) and nucleocapsid (N) proteins followed by RNA isolation and quantitative RT‐PCR (qRT‐PCR) using RNA‐dependent RNA polymerase (RdRp) and nucleocapsid (N) primers. Finally, a reporter assay was performed using SARS‐CoV‐2 pseudovirus.
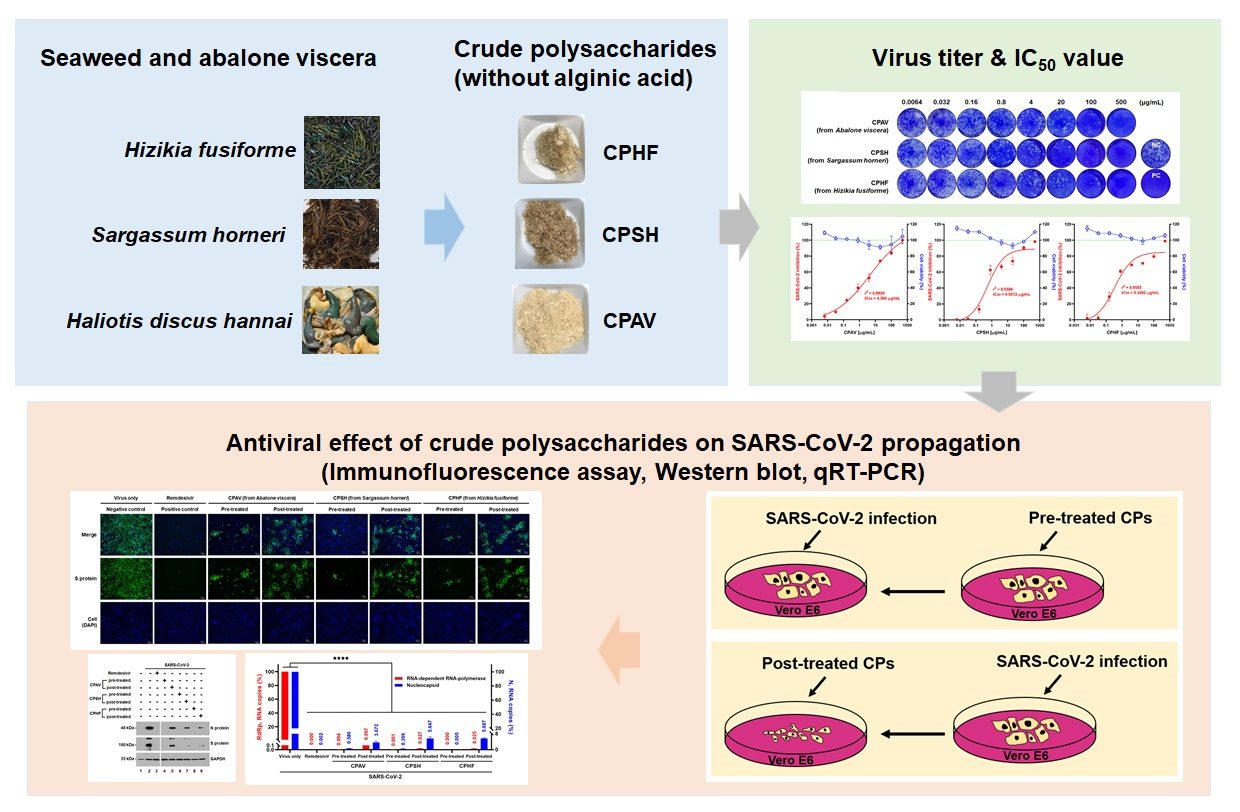
Study findings
The results indicated that the crude polysaccharide from abalone viscera was a little more toxic as compared to the other two CPs. Plaque formation was observed to be reduced in a dose-dependent manner by CPs, with 500μg/mL of CPs reducing plaque formation by 98 percent. Plaque formation was reported to be reduced by over 60 percent by CPHF and CPSH and 40 percent by CPAV for cells treated with 0.8 μg/mL CP concentrations before SARS‐CoV‐2 infection. Additionally, the concentrations with 50 percent inhibitory concentration (IC50) of CPHF, CPSH, and CPAV were observed to be 0.35, 0.56, and 4.37 μg/mL, respectively.
The crude polysaccharides were found to inhibit SARS-CoV-2 infection at 3 days and 4 days post-infection. The infectivity of SARS-CoV-2 in the presence of 500 μg/mL of CPAV, CPSH, and CPHF before the cells were treated with the virus was 16.5 ± 2.5 percent, 9.5 ± 3.2 percent, and 8.7 ± 2.7 percent. At the same time, it increased to 47.8 ± 6.6 percent, 48.7 ± 3.3 percent, and 45.7 ± 1.4 percent when cells were treated with CPs following virus infection.
The results also indicated that cells treated with CPs before virus infection did not express the viral S and N proteins. However, their expression was observed in cells treated with CPs after virus infection. Moreover, RdRp and N RNA copies were not detected in cells pre-treated with CPs while RdRp and N RNA copies were slightly increased in cells treated with CPs after infection. Also, SARS‐CoV‐2 pseudovirus cell entry was found to be strongly inhibited when pre-treated with CPs. However, the inhibitory effect was low when post‐treating with CPs.
Therefore, the current study demonstrated that SARS‐CoV‐2 propagation before and after viral infection could be inhibited by the crude polysaccharides of seaweed and abalone viscera. These crude polysaccharides need to be further purified and characterized to be used to develop therapeutics and prophylactics against the spread of the virus. Additional studies are required to examine the pharmacokinetics, perform nonclinical trials, and clarify the antiviral mechanisms when the structures of the marine polysaccharides are analyzed.
- Kang, S.M. et al. (2022). Evaluation of Antiviral Effect against SARS‐CoV‐2 Propagation by Crude Polysaccharides from Seaweed and Abalone Viscera In Vitro. Marine Drugs. Doi: https://doi.org/10.3390/md20050296. https://www.mdpi.com/1660-3397/20/5/296.
Posted in: Medical Science News | Medical Research News | Disease/Infection News
Tags: ACE2, Assay, Carotenoid, Cell, Coronavirus, Coronavirus Disease COVID-19, covid-19, Cytotoxicity, Drugs, Healthcare, in vitro, Nasal Spray, Pandemic, Pharmacokinetics, Polymerase, Propagation, Protein, Pseudovirus, Receptor, Respiratory, RNA, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Therapeutics, Tissue Culture, Vaccine, Virus

Written by
Suchandrima Bhowmik
Suchandrima has a Bachelor of Science (B.Sc.) degree in Microbiology and a Master of Science (M.Sc.) degree in Microbiology from the University of Calcutta, India. The study of health and diseases was always very important to her. In addition to Microbiology, she also gained extensive knowledge in Biochemistry, Immunology, Medical Microbiology, Metabolism, and Biotechnology as part of her master's degree.
Source: Read Full Article