Newborn babies who experience low levels of oxygen in their body tissues (hypoxia) due to sleep apnea, for example, tend to develop respiratory problems and hypertension (high blood pressure) in adulthood, and these problems may persist for the rest of their lives.
Research by scientists at São Paulo State University (UNESP) in Brazil shows for the first time that hypertension in these cases is caused by dysregulation of the autonomic nervous system, which controls involuntary physiological processes such as heart rate and breathing, as well as blood pressure.
An article on the study is published in the journal SLEEP.
The study involved an animal model and demonstrated that hypertension was associated with hyperactivity of the neurons in the sympathetic nervous system, the branch of the autonomic nervous system that is activated in response to stress.
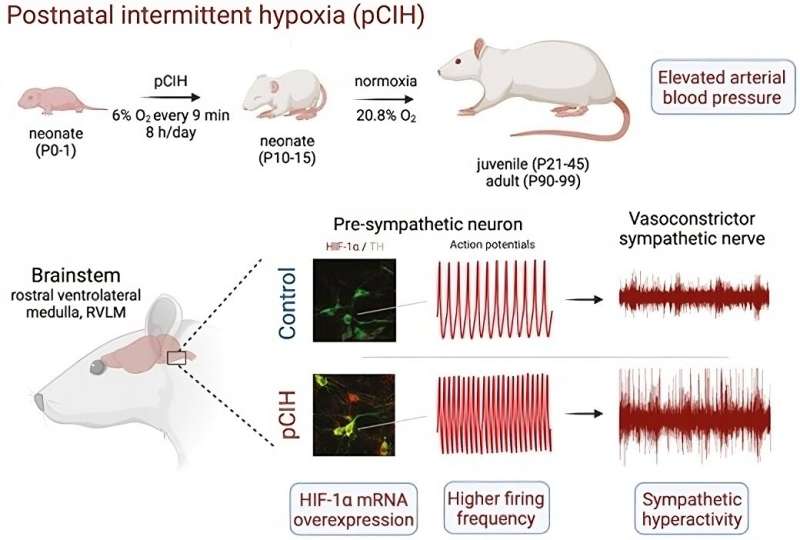
“We discovered that rats that experienced episodes of intermittent hypoxia in the postnatal period displayed higher levels of neuronal activity in the lower part of the brainstem [i.e. the medulla oblongata] in youth and adulthood.
This may reflect adaptations of the brain to low levels of oxygen during a critical stage of development, including heightened activity of the sympathetic autonomic nervous system, probably due to increased expression of a protein called hypoxia-inducible factor 1 alpha [HIF-1α] in medulla neurons,” said Daniel Zoccal, last author of the article and a professor at the Araraquara Dental School (FOAr-UNESP).
According to Zoccal, increased expression of HIF-1α by medulla neurons leads to several alterations in the “reading” of other genes that control cellular activity. As a result, the neurons that express more HIF-1α become more active, leading to a narrowing of the blood vessels and higher blood pressure. This phenomenon is epigenetic, insofar as it is due to biochemical alterations in cells caused by environmental stimuli that activate or silence genes without changing the genome.
Besides the first-ever demonstration of the mechanisms involved in the association between sporadically low levels of oxygen in the postnatal period and hypertension in youth and adulthood, important clinical developments could be based on the research.
“Although the prevalence of hypertension is high at about 30% of the world population, its origins are still poorly understood. All we know is that there’s a risk associated with factors such as obesity, sedentary lifestyles, kidney problems and consumption of salt, for example. The results of our study will serve as a basis for the development of novel therapies,” Zoccal told Agência FAPESP.
The discovery also highlights the importance of experiences during infancy to the development of diseases. “We must take greater care with babies’ breathing to prevent the development of disease in adulthood,” he stressed. Episodes of sleep apnea are fairly common in newborns and can be more frequent in premature infants, whose central nervous systems and respiratory systems are not fully mature, babies with enlarged adenoids or tonsils, obese infants, and babies with other anatomical abnormalities.
Describing the entire mechanism behind hypertension due to hypoxia in human infants up to about two years of age can also contribute to the development of treatments for the 20% or so of patients with hypertension who do not respond well to anti-hypertensive drugs, Zoccal said.
Previous research showed that electrical activity in the interface between sympathetic nerves and blood vessels is heightened in patients with high blood pressure, and especially those who do not respond to medicational treatment. “They have narrower blood vessels, which raises their blood pressure,” he said.
Postnatal chronic intermittent hypoxia
In the study, postnatal chronic intermittent hypoxia was induced in rats for the first ten days after birth. The hypoxic episodes were brief, with oxygen falling from 21% to 6% for 30 seconds every 10 minutes while they were asleep. This resulted in six episodes of sleep apnea per hour, equivalent to a case of moderate sleep apnea.
“In the clinic, there are cases of severe apnea where the patient experiences 30 or even 60 episodes per hour,” Zoccal said.
After two weeks, the researchers stopped inducing hypoxia, and the animals were able to breathe normally. When the rats were 40 days and 90 days old—comparable to between 13 and 16 years old and between 40 and 50 years old in humans—the researchers measured physiological parameters such as blood pressure and heart rate.
Rats of both ages subjected to postnatal intermittent hypoxia displayed consistently higher blood pressure—between 10 and 20 millimeters of mercury (mmHg) above the control group. According to the results, blood pressure in young rats averaged 84±7 mmHg for the control group and 95±5 mmHg for the hypoxia group. For adult rats, the averages were 103±10 mmHg and 121±9 mmHg respectively. It should be pointed out that blood pressure levels are similar in rats and humans.
“In the study, we didn’t analyze when animals became hypertense but confirmed that young rats already displayed alterations relating to blood pressure and were hypertense in adulthood,” Zoccal said.
After concluding that intermittent hypoxia raised blood pressure, the researchers set out to investigate the sympathetic nervous system’s contribution to this process.
A word of explanation is in place here. As noted earlier, the sympathetic nervous system is one branch of the autonomic nervous system. The other is the parasympathetic nervous system.
The sympathetic nervous system helps the body activate its “fight or flight” response to danger or a threat. Energy expenditure increases as it raises the heart rate and blood pressure, releases adrenalin, contracts and relaxes muscles, dilates the bronchi and pupils, accelerates respiration, and activates the sweat glands. The parasympathetic nervous system normalizes the internal organs once the threat has passed.
When they placed electrodes in contact with sympathetic nerves in young rats, the researchers observed more electrical impulses in the animals subjected to intermittent hypoxia than the control group. In adult rats, they used a pharmacological method that led to the same result as the tests involving young rats. “We administered a drug that inhibits the actions of the sympathetic nervous system, and depending on the drop in blood pressure, it was possible to infer that sympathetic activity was augmented,” Zoccal said.
The researchers also analyzed neuronal activity in the medulla oblongata, the bottom of the brainstem, responsible for regulating autonomic functions such as sneezing, breathing and heart rate, as well as sympathetic vasomotor tone and blood pressure.
“Our analysis focused on the ventral surface of the medulla, a key region for sympathetic activity to keep blood pressure at normal levels [about 12/8 mmHg in humans]. We found a higher firing rate for neurons in this region among the animals that had experienced postnatal intermittent hypoxia, pointing to dysfunction in this portion of the medulla due to exposure to hypoxia, which keeps sympathetic activity higher and raises blood pressure,” Zoccal said.
They also found that neurons in the sympathetic nervous system expressed more HIF-1α. “This discovery enabled us to associate the entire process with a possible epigenetic cause,” he said.
Zoccal recalled that the 2019 Nobel Prize in Physiology or Medicine was awarded to three scientists for their discoveries of how cells sense and adapt to oxygen availability, and that studies of HIF-1α were a significant part of their work. They discovered that levels of the protein increase when oxygen is low, inducing adaptations that assure the survival of cells and the organism during hypoxic conditions. Levels of HIF-1α decline when the supply of oxygen is normal.
The study focused on the effect of postnatal intermittent hypoxia on blood pressure due to sympathetic nervous system dysfunction, but alterations in this system are known to bring about other modifications, since sympathetic activity controls many functions in the organism, including body temperature and hence metabolism.
“In another study we published, using the same experimental model, the body weight of the animals that experienced hypoxia was lower than that of the control group, possibly owing to increased sympathetic activity. We also noted respiratory irregularities, with an abnormal pattern of acceleration and deceleration of resting pulmonary ventilation. In sum, these animals not only have high blood pressure but may also suffer from respiratory problems and probably from metabolic alterations as well,” Zoccal said.
More information:
Marlusa Karlen-Amarante et al, Sympathetic dysregulation induced by postnatal intermittent hypoxia, SLEEP (2023). DOI: 10.1093/sleep/zsad055
Journal information:
Sleep
Source: Read Full Article