Your brain is powered by 400 miles of blood vessels that provide nutrients, clear out waste products, and form a tight protective barrier—the blood brain barrier—that controls which molecules can enter or exit. However, it has remained unclear how these brain vascular cells change between brain regions, or in Alzheimer’s disease, at single-cell resolution.
To address this challenge, a team of scientists from MIT’s Computer Science and Artificial Intelligence Laboratory (CSAIL), The Picower Institute for Learning and Memory, and The Broad Institute, recently unveiled a systematic molecular atlas of human brain vasculature and its changes in Alzheimer’s Disease (AD) across six brain regions, in a paper published in Nature Neuroscience.
Alzheimer’s Disease is a leading cause of death, affects one in nine Americans over 65, and leads to debilitating and devastating cognitive decline. Impaired blood brain barrier (BBB) function has long been associated with Alzheimer’s and other neurodegenerative diseases such as Parkinson’s and multiple sclerosis. However, the molecular and cellular underpinnings of BBB dysregulation remain ill-defined, particularly at single-cell resolution across multiple brain regions and many donors.
Navigating vascular complexity
Embarking deep into the complexities of our gray matter, the researchers created a molecular atlas of human brain vasculature across 428 donors, including 220 diagnosed with Alzheimer’s and 208 controls. They characterized over 22,514 vascular cells from six different brain regions, measuring the expression of thousands of genes for each cell. The resulting datasets unveiled intriguing changes in gene expression across different brain regions, and stark contrasts between individuals afflicted with AD and those without.
“Alzheimer’s therapy development faces a significant hurdle—brain alterations commence decades before cognitive signs make their debut, at which point, it might already be too late to intervene effectively,” comments MIT CSAIL principal investigator and MIT EECS Professor Manolis Kellis, the study’s senior author. “Our work charts the terrain of vascular changes, one of the earliest markers of Alzheimer’s, across multiple brain regions, providing a map to guide biological and therapeutic investigations earlier in disease progression.”
The little cells that could
The threads of our human brain vasculature, and every part of our brain and body, is composed of millions of cells, all sharing the same DNA code, but each expressing a different subset of genes, which define its functional roles and distinct cell type. Using the distinct gene expression signatures of different cerebrovascular cells, the researchers distinguished 11 types of vascular cells.
These included endothelial cells that line the interior surface of blood vessels and control which substances pass through the BBB, pericytes that wrap around small vessels and provide structural support and blood flow control, smooth muscle cells that form the middle layer of large vessels and whose contraction and relaxation regulates blood flow and pressure, fibroblasts that surround blood vessels and hold them in place, and they distinguished arteriole, venule, and capillary veins responsible for the different stages of blood oxygen exchange.
The abundance of these vascular cell types differed between brain regions, with neo-cortical regions showing more capillary endothelial cells and fewer fibroblasts than subcortical regions, highlighting the regional heterogeneity of the BBB.
Clues and suspects
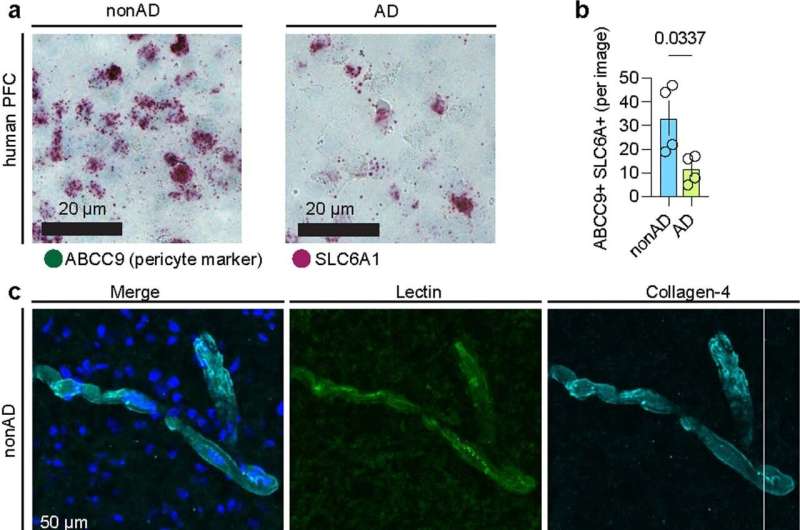
Armed with these annotations, the next phase was studying how each of these cell types change in AD, revealing 2,676 genes whose expression levels change significantly. They found that capillary endothelial cells, responsible for transport, waste removal, and immune surveillance, showed the most changes in AD, including genes involved in clearance of Amyloid beta, one of the pathological hallmarks of AD, providing insights on the potential mechanistic implications of vascular dysregulation on AD pathology.
Other dysregulated processes included immune function, glucose homeostasis, and extracellular matrix organization, which were all shared among multiple vascular cell types, and also cell-type-specific changes, including growth factor receptors in pericytes, and transporter and energy in endothelial cells, and cellular response to Amyloid beta in smooth muscle cells. Regulation of insulin sensing and glucose homeostasis in particular suggested important connections between lipid transport and Alzheimer’s regulated by the vasculature and blood-brain-barrier cells, which could hold promise for new therapeutic clues.
“Single-cell RNA sequencing provides an extraordinary microscope to peer into the intricate machinery of life, and ‘see’ millions of RNA molecules bustling with activity within each cell,” says Kellis, who is also a member of the Broad Institute. “This level of detail was inconceivable just a few years ago, and the resulting insights can be transformative to comprehend and combat complex psychiatric and neurodegenerative disease.”
Maestros of dysregulation
Genes do not act on a whim, and they do not act alone. Cellular processes are governed by a complex cast of regulators, or transcription factors, that dictate which groups of genes should be turned on or off in different conditions, and in different cell types. These regulators are responsible for interpreting our genome, the ‘book of life,” and turning into the myriad of distinct cell types in our bodies and in our brains. These regulators might be responsible when something goes wrong, and they could also be critical in fixing things and restoring healthy cellular states.
With thousands of genes showing altered expression levels in Alzheimer’s Disease, the researchers then sought to find the potential masterminds behind these changes. They asked if common regulatory control proteins target numerous altered genes, which may provide candidate therapeutic targets to restore the expression levels of large numbers of target genes. Indeed, they found several such ‘master controllers,” involved in regulating endothelial differentiation, inflammatory response and epigenetic state, providing potential intervention points for drug targets against AD.
Cellular murmurings
Cells do not function in isolation; rather, they rely on communication with each other to coordinate biological processes. This intercellular communication is particularly complex within the cellular diversity of the brain, given the many factors involved in sensing, memory formation, knowledge integration, and consciousness. In particular, vascular cells have intricate interactions with neurons, microglia, and other brain cells, which take on heightened significance during pathological events, such as in Alzheimer’s disease, where dysregulation of this cellular communication can contribute to the progression of the disease.
They found that interactions from capillary endothelial cells to neurons, microglia, and astrocytes were highly increased in AD, while interactions in the reverse direction, from neurons and astrocytes to capillary endothelial cells, were decreased in AD. This asymmetry could provide important cues for potential interventions targeting the vasculature and specifically capillary endothelial cells, with ultimate broad positive impacts on the brain.
“The dynamics of vascular cell interactions in AD provide an entry point for brain interventions and potential new therapies,” says Na Sun, an MIT CSAIL and EECS graduate student and first author on the study. “As the blood brain barrier prevents many drugs from influencing the brain, perhaps we could instead manipulate the blood brain barrier itself, and let it spread beneficiary signals to the rest of the brain. Our work provides a blueprint for cerebrovasculature interventions in Alzheimer’s disease, by unraveling how cellular communication can mediate the impact of genetic variants in AD.”
Going off script: Genetic plot twists
Disease onset in our bodies (and in our brains) is shaped by a combination of genetic predispositions and environmental exposures. On the genetic level, most complex traits are shaped by hundreds of minuscule sequence alterations, known as single-nucleotide polymorphisms (or SNPs, pronounced snips), most of which act through subtle changes in gene expression levels.
No matter how subtle their effects might be, these genetic changes can reveal causal contributors to disease, which can greatly increase the chance of therapeutic success for genetically-supported target genes, compared to targets lacking genetic support.
To understand how genetic differences associated with Alzheimer’s might act in the vasculature, the researchers then sought to connect genes that showed altered expression in Alzheimer’s with genetic regions associated with increased Alzheimer’s risk through genetic studies of thousands of individuals. They linked the genetic variants (SNPs) to candidate target genes using three lines of evidence: physical proximity in the three-dimensional folded genome, genetic variants that affect gene expression, and correlated activity between distant regulatory regions and target genes that go on and off together between different conditions.
This resulted in not just one hit, but 125 genetic regions, where Alzheimer’s-associated genetic variants were linked to genes with disrupted expression patterns in Alzheimer’s disease, suggesting they might mediate these causal genetic effects, and thus may be good candidates for therapeutic targeting. Some of these predicted hits were direct, where the genetic variant acted directly on a nearby gene. Others were indirect, when the genetic variant instead affected the expression of a regulator, which then affected expression of its target genes. And yet others were predicted to be indirect through cell-cell communication networks.
ApoE4 and cognitive decline
While most genetic effects are subtle, both in Alzheimer’s and nearly all complex disorders, exceptions do exist. One such exception is FTO in obesity, that increases obesity risk by one standard deviation. Another one is apolipoprotein E (ApoE) in Alzheimer’s Disease, where the e4 vs. e3 allele increases risk more than 10-fold for carriers of two risk alleles—who inherited one “unlucky” copy from each parent.
With such a strong effect size, the researchers then asked if ApoE4 carriers showed specific changes in vascular cells that were not found in ApoE3 carriers. Indeed, they found abundance changes associated with the ApoE4 genotype, with capillary endothelial cells and pericytes showing extensive down-regulation of transport genes. This has important implications for potentially preventive treatments targeting transport in ApoE4 carriers, especially given the cholesterol transporter roles of ApoE, and the increasingly recognized role of lipid metabolism in Alzheimer’s DIsease.
“Unearthing these AD-differential genes gives us a glimpse into how they may be implicated in the deterioration or dysfunction of the brain’s protective barrier in Alzheimer’s patients, shedding light on the molecular and cellular roots of the disease’s development,” says Kellis. “They also open several avenues for therapeutic development, hinting at a future where these entry points might be harnessed for new Alzheimer’s treatments targeting the blood brain barrier directly. The possibility of slowing or even halting the disease’s progression is truly exciting.”
Translating these findings into viable therapeutics will be a journey of exploration, demanding rigorous preclinical and clinical trials. To bring these potential therapies to patients, the scientists need to understand how to target the discovered dysregulated genes safely and effectively, and determine whether modifying their activity can ameliorate or reverse AD symptoms, which requires extensive collaborations between medical doctors and engineers across both academia and industry.
More information:
Na Sun et al, Single-nucleus multiregion transcriptomic analysis of brain vasculature in Alzheimer’s disease, Nature Neuroscience (2023). DOI: 10.1038/s41593-023-01334-3
Journal information:
Nature Neuroscience
Source: Read Full Article