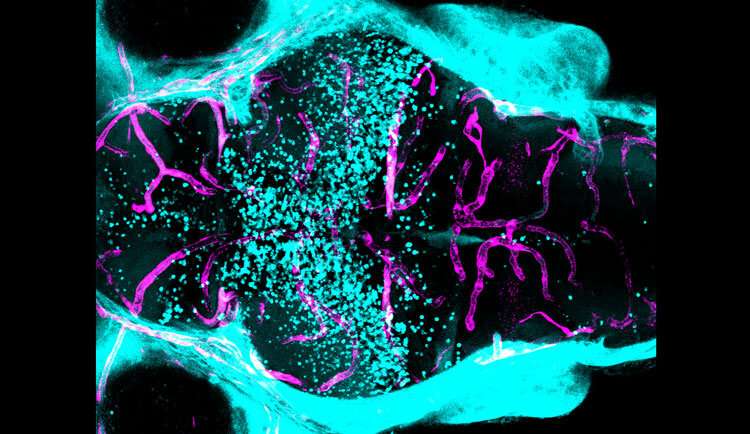
What makes the vital layer of protective cells around the brain and spinal cord—the blood-brain barrier—more or less permeable has been one of the more mystifying questions in neuroscience.
Understanding how the barrier works to allow in or keep out certain substances has critical implications for everything from disease progression to drug delivery.
Now, a new Harvard Medical School study, published July 11 in Developmental Cell, has brought scientists a step closer to figuring it out.
Working in zebrafish and mice, the team discovered that a signal originating from a gene in neurons is essential for the proper formation of the blood-brain barrier during embryonic development and helps ensure that the barrier remains intact throughout adulthood.
If replicated in further animal testing and eventually in humans, the findings could help scientists control the permeability of the blood-brain barrier. In doing so, researchers may be able to develop more effective ways of delivering cancer or psychiatric medicines into the brain and better strategies for combating barrier damage caused by neurodegeneration or stroke.
Following the science
The blood-brain barrier is made of tightly interlaced cells—endothelial cells, pericytes, and astrocytes—lining the blood vessels of the brain and spinal cord that make up the central nervous system. Together, these cells form a layered, semipermeable membrane that selectively lets in nutrients and small molecules, while keeping out harmful substances.
“In normal, day-to-day life, you need a blood-brain barrier to help protect you from invading toxins and pathogens in the blood,” explained lead author Natasha O’Brown, a research fellow in systems biology at HMS who is starting her lab at Rutgers University in September.
In the case of neurodegenerative diseases such as Alzheimer’s or Parkinson’s, or stroke, the barrier begins to break down, leaving the central nervous system susceptible to infection. On the flip side, the impermeability of the barrier presents an obstacle for delivering drugs to the brain.
For decades, scientists have known that the permeability of the blood-brain barrier is in part controlled by cells in the surrounding environment—known as the microenvironment. However, the genes in those nearby cells have largely remained a mystery.
Unbeknownst to the researchers, a major clue was swimming around inside fish tanks in the lab of senior author Sean Megason, professor of systems biology in the Blavatnik Institute at HMS.
O’Brown was studying a gene called mfsd2aa that, when mutated, causes the blood-brain barrier in zebrafish to become leaky throughout the entire brain. However, she noticed that some zebrafish had a barrier that was permeable in the forebrain and midbrain, but intact in the hindbrain.
“This observation led me down a rabbit hole of finding the gene that causes the blood-brain barrier to become regionally permeable,” she said.
A new character emerges
O’Brown conducted genetic screens on the zebrafish and discovered that the region-specific breakdown of the barrier was linked to a mutation in spock1—a gene whose name brought to mind the Star Trek character but was otherwise unfamiliar to her.
In a series of experiments in zebrafish and mice, O’Brown confirmed that a spock1 mutation caused the blood-brain barrier to become permeable in some areas but not others. She also saw that spock1 was expressed in neurons throughout the retina, brain, and spinal cord, but not in the cells that make up the barrier itself.
In follow-up experiments, animals with a spock1 mutation had more vesicles—intercellular bubbles that can carry large molecules across the blood-brain barrier—in their endothelial cells. They also had a smaller basement membrane, a network of proteins found between endothelial cells and pericytes in the barrier. Cell-by-cell RNA analysis revealed that spock1 caused changes in gene expression in endothelial cells and pericytes in the blood-brain barrier, but not in other cell types in the brain. When O’Brown injected a dose of human SPOCK1 protein into zebrafish brains, it restored around 50 percent of blood-brain barrier function by repairing pericyte–endothelial cell interactions at a molecular level.
Based on these findings, the researchers concluded that the Spock1 protein produced by neurons travels to the blood-brain barrier, where it initiates the proper formation of the barrier during development and helps maintain the barrier after.
“Spock1 is a potent secreted neural signal that is able to promote and induce barrier properties in these blood vessels; without it, you don’t get a functional blood-brain barrier,” O’Brown said. “It’s like a spark on a gas stove, providing a cue that tells the barrier program to turn on.”
Completing the picture
The study adds to a growing body of research by renowned blood-brain barrier biologist Chenghua Gu, professor of neurobiology at HMS, investigator at the Howard Hughes Medical Institute, and an author on the new paper. Her lab has been studying a cellular trafficking system that seems to regulate blood-brain barrier permeability through Mfsd2a, and exploring other aspects of the microenvironment that may be involved. Cumulatively, the work is providing scientists with an increasingly complete picture of how the blood-brain barrier functions.
Gaining this complete picture is essential as researchers attempt to manipulate the permeability of the barrier. For drug delivery, they often want to make the barrier more permeable, so therapies known to be effective for cancer or psychiatric disorders can reach the brain and do their jobs. For neurodegenerative diseases such as Parkinson’s and Alzheimer’s or situations like stroke, scientists want to counter the associated deterioration of the blood-brain barrier that makes the central nervous system vulnerable to external assaults.
O’Brown noted that spock1 is an especially appealing target for controlling the properties of the blood-brain barrier because it is conserved in humans and seems to act as a high-level regulator of barrier cells during development.
She now wants to explore how different lineages of pericytes in the barrier are differentially affected by spock1 signaling. She would also like to test out stroke models, to see if administering spock1 can counter a stroke’s effects on the blood-brain barrier.
“This isn’t the first neural signal scientists have found, but it is the first signal from neurons that specifically seems to regulate barrier properties,” O’Brown said. “I think this makes it a potent tool to try and toggle the switch.”
Additional authors on the paper include Nikit Patel and Allon Klein of HMS, and Ursula Hartmann of the University of Cologne.
More information:
Natasha M. O’Brown et al, The secreted neuronal signal spock1 promotes blood-brain barrier development, Developmental Cell (2023). DOI: 10.1016/j.devcel.2023.06.005
Journal information:
Developmental Cell
Source: Read Full Article