Minks may be a suitable animal model for testing severe COVID-19 infections, finds a recent bioRxiv* preprint study. While mice and ferrets are used in preclinical severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), there is currently no model specific to severe disease.
Farmed minks are naturally infected with SARS-CoV-2 and can spread the virus to humans. Infected minks not only develop an infection, but they also exhibit similar severe symptoms seen in humans, such as severe acute respiratory disease. The data shows high amounts of viral RNA are detected from the respiratory tract.
Testing new antiviral treatments to suppress viral spread or prevent infection may help lower the number of people requiring hospitalization.
SARS-CoV-2 enters mink cells via ACE2 receptor
SARS-CoV-2 invades human cells by binding with the ACE2 receptor. The research team compared the viral entry in humans to minks and whether the mechanism for viral entry was similar.
The team collected a sequence of ACE2 from the lung tissue of the American mink. The American and European mink had 99.9% identical sequences. For humans, there was an approximate 83% similarity.
There were differences in SARS-CoV-2’s binding residues between minks and humans, which interact with ACE2. A 65% similarity was observed for the binding residues between mink and human ACE2. Seven of 20 interface residues differed in mink. Binding residues that have previously been shown to be critical for the interaction of ACE2 and the spike protein — K31, Y41, and Y353 — were conserved.
The changes to human and mink ACE2 did not significantly differ in how the virus enters the cell. They found this result after injecting fibroblasts with either human or mink ACE2 and exposing the cells to SARS-CoV-2.
There was significantly increased viral entry in mink ACE2 expressing cells than human ACE2 expressing cells when exposed to the WA1 lineage A SARS-CoV-2 spike protein. However, when cells were exposed to the Alpha variant, there were no differences in the viral entry between humans and minks cells.
Minks develop severe COVID-19 infection 2 days after exposure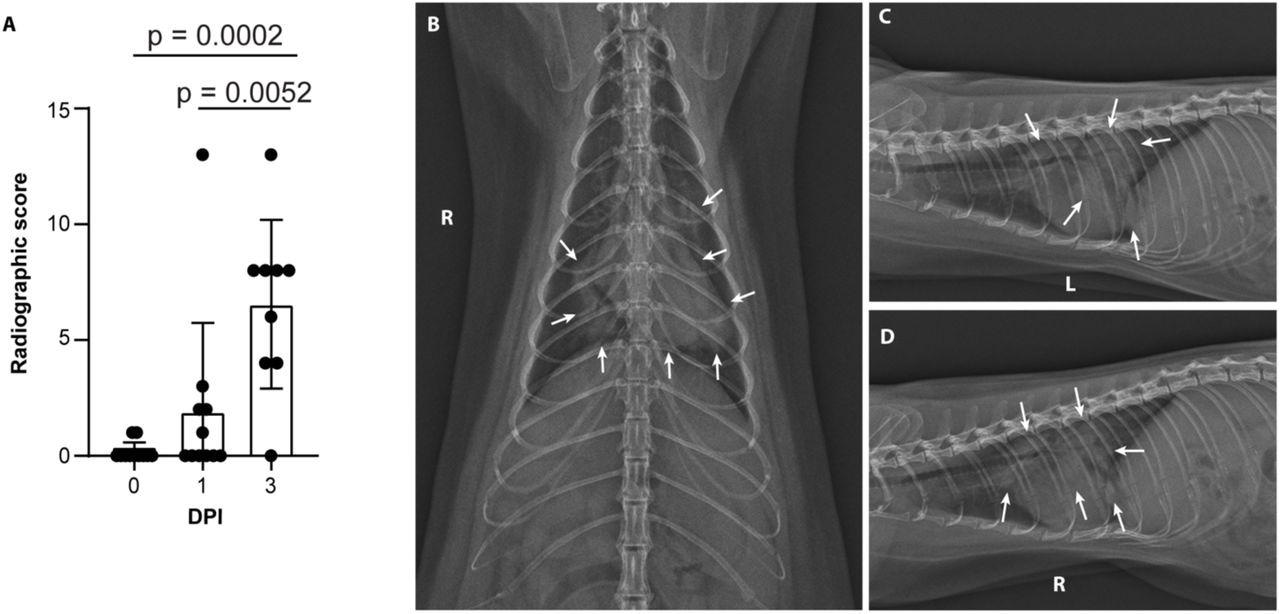
Severe radiological changes after infection with SARS-CoV-2. (A) Compiled radiographic scores. Bar graph depicts the mean with standard deviation and individuals, ordinary one-way ANOVA with Tukey’s multiple comparisons test. Radiographs demonstrate multifocal pulmonary infiltrates, most severe in the left and right caudal lung lobes depicted in the (B) dorsoventral radiograph (C) left lateral and (D) right lateral radiograph on the evening of 2 days post-inoculation (DPI). Arrows depict grade 4 pulmonary disease in the left and right caudal lung lobes with grade 3 pulmonary disease in the right middle lung lobe and cranial subsegment of the left cranial lung lobe.
Eleven adult minks were administered the Alpha variant where two died 2 days later, eight died 3 days later, and one survived for 28 days before being euthanized.
All animals experienced a 15% reduction in weight 3 days after viral exposure. For the animal that later recovered, their body weight returned to preinfection levels after 14 days.
Five of the 11 minks showed their first signs of COVID-19 infection 1 day after exposure. By the second day, 9 of the 11 had symptoms. All animals were symptomatic by day 3.
Minks developed severe COVID-19 symptoms including, dull mentation, shivering, hunched or balled posture, lethargy, anorexia, increased respiratory effort, abnormally rapid breath, and nasal discharge.
On an immune level, the researchers observed a decrease in white blood cell count from mild lymphopenia. In addition, the neutrophil-to-lymphocyte ratio was significantly higher for animals severely ill. The animal which later recovered from infection had an elevated neutrophil-to-lymphocyte ratio that peaked 5 days after exposure before decreasing.
Minks developed progressive pulmonary infiltrates consistent with viral pneumonia from ARDS on the first and third day after exposure.
All minks were euthanized, and their bodies were examined to look at the damage caused by the virus. They found 100% of the lungs were affected as they were considered hyperemic. In addition, some animals had pulmonary hepatization. Much lung damage resembled those seen in humans with severe COVID-19 pulmonary damage and coagulopathy.
Nasal cavities of minks had rare neutrophilic infiltration of the olfactory epithelium and multifocal fibrin thrombi in regions of congestion.
Viral RNA levels detected one day after exposure
Viral shedding was found in all animals as early as 1 day after exposure. The highest viral RNA loads were in the oral and nasal swabs.
Nasal, oral, and rectal swabs had sub-genomic RNA, a marker of viral replication, and that minks were infectious.
In the animal that recovered from infection 28 days after exposure, viral RNA and signs of an infectious virus were found until day 7 in nasal swabs and day 10 in oral swabs.
The highest viral load was found in respiratory tissues — especially in the upper and lower tract.
Mutations from SARS-CoV-2 genomes were also observed, suggesting the potential to study new variants.
*Important notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Adney DR, et al. (2022). Severe acute respiratory disease in American mink (Neovison vison) experimentally 2 infected with SARS-CoV-2. bioRxiv. Doi: https://doi.org/10.1101/2022.01.20.477164, https://www.biorxiv.org/content/10.1101/2022.01.20.477164v1
Posted in: Medical Science News | Medical Research News | Disease/Infection News
Tags: ACE2, Animal Model, Anorexia, Blood, Cell, Coronavirus, Coronavirus Disease COVID-19, Genomic, Lethargy, Lungs, Lymphocyte, Lymphopenia, Pneumonia, Posture, Preclinical, Protein, Receptor, Research, Respiratory, Respiratory Disease, RNA, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Virus, White Blood Cell

Written by
Jocelyn Solis-Moreira
Jocelyn Solis-Moreira graduated with a Bachelor's in Integrative Neuroscience, where she then pursued graduate research looking at the long-term effects of adolescent binge drinking on the brain's neurochemistry in adulthood.
Source: Read Full Article