It’s no secret that our lungs play a vital role in our daily lives—ensuring we can breathe, fend off infections, and adapt to various challenges. Despite their importance, the organs still puzzle many medical experts, especially when they’re affected by diseases. While traditional tools like MRI and CT scans are helpful when a patient is experiencing a lung-related illness, they can still fall short in providing the detailed, real-time information needed to understand the intricacies of lung health.
Enter the groundbreaking innovation known as the crystal ribcage. Developed by researchers in Boston University’s College of Engineering, Pulmonary Center, Center for Multiscale and Translational Mechanobiology, and Neurophtonics Center, the technology is poised to revolutionize not only our understanding of lung function but also holds immense potential for other organs and treatments.
In new research, published this month in Nature Methods, the crystal ribcage acts as a clear, protective shield for a mouse’s lungs, allowing scientists to get a close view of how these organs work in real-time, and at a cellular level. What makes this technology special is that it doesn’t disrupt the lung’s natural processes—breathing and blood circulation continue as usual while the researchers observe.
In this Q&A, senior author Dr. Hadi Nia discusses the crystal ribcage, how it’s reshaping our understanding of lung research, and its potential uses beyond the lungs.
What is the primary challenge highlighted in the research when it comes to understanding lung health?
The lung is the site of many fatal pathologies such as primary and metastatic cancers, respiratory infections and both obstructive and restrictive diseases that impact its functions at the cellular level. With existing imaging modalities such as MRI, CT, and histological analyses, dynamic single cell events at the early stages of disease progression, such as the interactions of immune cells with cancer cells and bacteria, cannot be resolved.
With the crystal ribcage, we can for the first time image the mouse lung down to cellular levels while it retains its physiological functions such as respiration, circulation of blood, and immune activity. We are now able to study many lung diseases at the earliest steps of disease initiation.
How does the crystal ribcage differ from traditional methods like MRI and CT scans when studying the lungs?
MRI and CT allow the visualization of the whole lung, but their spatial and temporal resolution are low, and single air sacs (known as alveolus), single capillaries, and cellular events such as migration of immune and cancer cells cannot be resolved. Crystal ribcage on the other hand, allows the use of optical microscopy by replacing the actual ribcage made from bone and muscle and hence blocking the light, by a transparent rib made form biocompatible materials.
Since crystal ribcage has the same geometry and material properties of the actual rib, the lung can maintain its physiological functions as being imaged by an optical microscope. If combined with a fast enough microscope, the dynamic events such as cellular trafficking and blood flow can be imaged in real-time when the lung is in action. This technology basically opens the black box of the lung in health and disease, and provides real-time views of the lung that were never seen before.
Can you describe how the crystal ribcage works to enable close observation of a mouse’s lung?
The crystal ribcage design is informed from the microCT of the native mouse ribcage to ensure that the lung can function physiologically inside the crystal ribcage. The lung inside crystal ribcage is ventilated either by positive-pressure to simulate mechanical ventilation, or with negative-pressure to simulate spontaneous breathing. The lung is also circulated with media or blood to provide nutrients to the lung cells. The crystal ribcage is then placed on the microscope stage and the lung is imaged at the resolution of interest.
In addition to the imaging capabilities that crystal ribcage provides, we are able to intervene in many ways in the respiration or circulation, such as adding drugs, or changing the lung physiological parameters (e.g., simulating exercise by increasing breathing rate and depth), and study the subsequent changes in the lung structure-function. This controllability when combined with imaging capabilities make crystal ribcage a transformative technology to study many key lung diseases.
In addition to lung research, what other organs might benefit from the application of the crystal ribcage technology?
We have used the crystal ribcage to image the heart in conjunction with the lung at elevated vascular pressures. Such experiments will allow further probing of circulation-respiration coupling in disease such as pulmonary hypertension or arrhythmia in the future. With the success and enthusiasm that we have generated, future directions in Nia Lab include translating similar ideas to the brain by fabricating a “crystal skull” to visualize the whole brain in action.
How might the crystal ribcage technology advance studies related to organ transplants and regenerative medicine?
Crystal ribcage advances these studies in two ways:
- Providing a physiological environment for the transplant or engineered tissue where the sample can be imaged at the cellular resolution in real-time.
- Allowing intervention by changing the physiological parameters, administration of therapeutic agents, and monitoring the subsequent alteration in the lung.
As one example, there is active research on how long we can keep the human lung viable in between the donor-receiver surgeries. This time, currently at 6–8 hours, if extended, can be extremely invaluable as it allows storing and transporting the organ to other transplantation sites. Our crystal ribcage allows monitoring the ex vivo lung at the optical resolution, and hence allows the researchers to better understand why the human lung cannot be maintained outside the body for more than 6–8 hours.
As another example, there is an active line of research on lung tissue engineering. Despite the progress on engineering the lung, there is no reliable way to evaluate the function of the lung. Crystal ribcage allows this evaluation at the cellular level while allowing the engineered lung function in a physiological environment.
How does the crystal ribcage contribute to a better understanding of lung diseases, development, and aging?
The crystal ribcage allows three-dimensional (3D) imaging over the entire surface of the lung, while uniquely combining the benefits of in vivo mouse models (cellular diversity, 3D lung architecture, breathing and circulation) and in vitro organ-on-chip models (imaging advantages and extensive, precise control over microphysiology). We basically can see how disease progression and aging affect the lung structure-function at single cell resolution and in real-time.
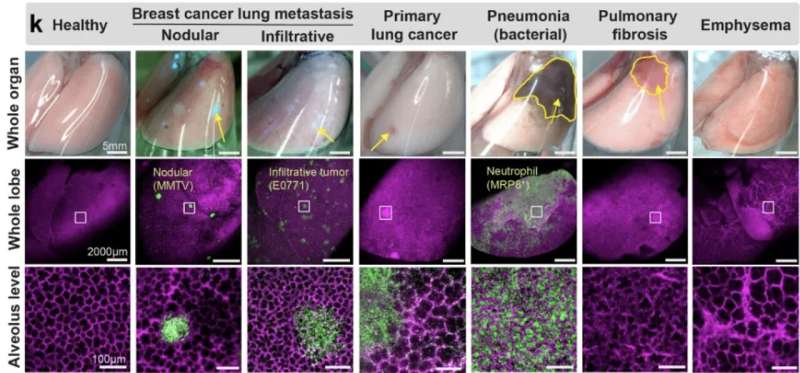
As examples, we utilized the crystal ribcage to probe remodeling of alveolar and capillary functions in mouse models of primary and metastatic lung cancer, bacterial infection, pulmonary fibrosis, emphysema, and acute lung injury. We identified the earliest stage of tumorigenesis when alveolar structure–functions are compromised, probed the dynamic circulatory functions of capillaries remodeled by tumor growth and pneumonia and demonstrated a dramatic and reversible mechano-responsiveness in immune cell migration in the lung.
As another example, it is well known that aging is major risk factor for pneumonia. However, we do not know exactly why. In collaboration with Joseph Mizgerd, the Directory of Pulmonary Center, we are now utilizing crystal ribcage to better understand how aging may affect the pneumonia progression or resolution.
This is all but a tip of the iceberg and we are excited to collaborate with the research community to further our collective knowledge of disease progression, therapeutic effects, and aging of the lung as it functions.
Who are your key research collaborators?
Our research collaborators span both the Charles River and Medical campuses and are part of the BU Pulmonary Center, BU Center for Multiscale and Translational Mechanobiology, and BU Neurophtonics Center. Key investigators who supported our research are Dr. Bela Suki, Dr. Joseph Mizgerd, Dr. Sarah Mazilli, Dr. Katrina Traber, and Dr. Giovanni Ligresti.
More information:
Rohin Banerji et al, Crystal ribcage: a platform for probing real-time lung function at cellular resolution, Nature Methods (2023). DOI: 10.1038/s41592-023-02004-9
Journal information:
Nature Methods
Source: Read Full Article