No one truly knows the experience of suffering a disease better than the patient. Yet until the 1990s, medical research was largely something done on, and for, patients. Led by movements in the US and UK, patients are now increasingly becoming active collaborators and shared decision-makers in research.
A new study from Osaka University shows Japan also may be ready to embrace, and make great discoveries through, patients’ research involvement.
The Rare and Undiagnosed Diseases Study (RUDY) JAPAN officially launched in December 2017. It was born from a collaboration between Osaka University and the University of Oxford. RUDY JAPAN, following in the footsteps of RUDY UK, sought to clarify what types of active patient involvement and collaborative research would fit into Japan’s research ethics and regulations, as well the country’s culture.
A retrospective of the study was published in the journal, Research Involvement and Engagement.
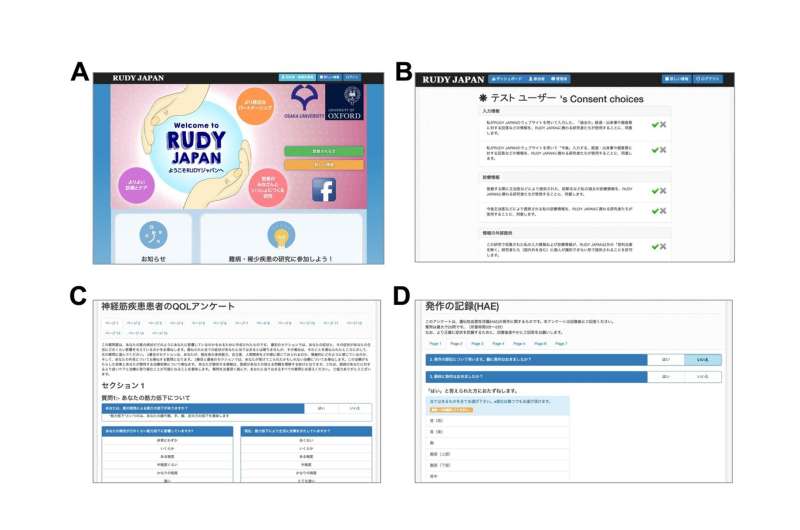
RUDY provides an online platform for patients to track their disease outcomes through questionnaires and record-keeping. It also includes a dynamic consent function for patients to manage their participation and data, and for research to satisfy ethical requirements. RUDY JAPAN focused first on neurological dysfunctions called channelopathies, and later on hereditary angioedema, which causes unpredictable swelling attacks.
“In the last few years, Japan has become more open to patient involvement in research,” co-lead authors Nao Hamakawa and Atsushi Kogetsu say. “Rare diseases are a suitable target for such research as they have small populations, so it’s especially difficult to research their symptoms and patterns. The RUDY system’s Internet-based accessibility let us simultaneously research rare diseases and involve patients in the process.”
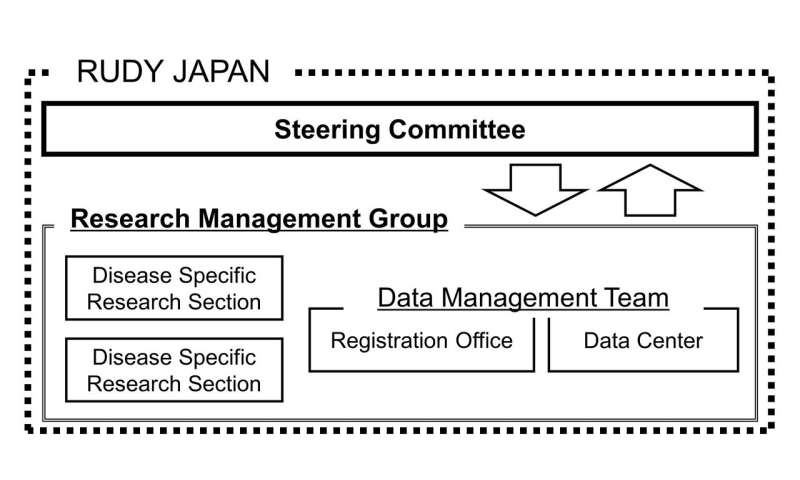
Patients took part in two ways. Patient partners joined a Steering Committee that decided on design and management aspects, such as compiling the questionnaires used in the research. Patient participants registered with the RUDY JAPAN system and reported on their conditions.
With surveys as a central aspect in many studies, patients’ input and direction was especially useful in the questionnaire development. Virtual meeting tools also applied ICT to facilitate interaction that may otherwise require time and travel.
“Both patients and researchers realize benefits through the RUDY JAPAN system,” corresponding author Kazuto Kato notes. “The researchers can see how the patients actually think about research, and how to communicate with them. Meanwhile, the patients can feel that the research is more relevant to their dairy life, and they have ways to track their symptoms.”
There are also some hurdles in the time and effort needed for this type of research, as well as the challenges of patients handling academic exchange.
Source: Read Full Article