The emerging advantages of positron-emission tomography (PET) myocardial perfusion imaging (MPI) for coronary artery disease (CAD) diagnosis and assessment of cardiovascular event risk has prompted growing use of this technology as an alternative to the more commonly used single photon–emission computed tomography (SPECT) MPI.
The advantages of PET MPI include better diagnostic performance and shorter acquisition times. The latest position statement from the American Society of Nuclear Cardiology and Society of Nuclear Medicine and Molecular Imaging highlights these advantages and pinpoints additional important properties of PET, including consistent, high-quality images and low radiation exposure, it allows quantification of myocardial blood flow, and it has “strong prognostic power.”
Tracer Availability
Despite these advantages, that position paper and subsequent studies note that PET MPI has been underutilized in the United States, largely owing to issues with the available tracers, which have characteristics that limit widespread use in the clinic.
Rubidium, arguably the most commonly used tracer for PET MPI, is not available in unit dosing and so can be expensive for low-volume centers, plus it also requires an on-site generator, Michael Salerno, MD, PhD, a member of the American College of Cardiology’s Imaging Council and section chief of cardiovascular imaging, Stanford University, California, told theheart.org | Medscape Cardiology.
N-ammonia, the other US Food and Drug Administration (FDA)–approved tracer, is available in unit dosing, but its short half-life means that centers need an onsite cyclotron, Salerno said.
For cardiac perfusion imaging and myocardial blood flow (MBF) quantification, 15O-water is considered the gold standard, although it’s not approved by the FDA. This tracer also requires an on-site cyclotron and “is challenging to use,” Salerno said. Use has been largely restricted to research purposes, though efforts are underway to widen its availability.
Enter flurpiridaz F-18 (GE Healthcare), a novel PET MPI tracer labeled with fluorine-18. Its longer half-life ― similar to that of fluorodeoxyglucose, a tracer used to detect various cancers ― could broaden the number of sites that could perform perfusion PET studies, Salerno said.
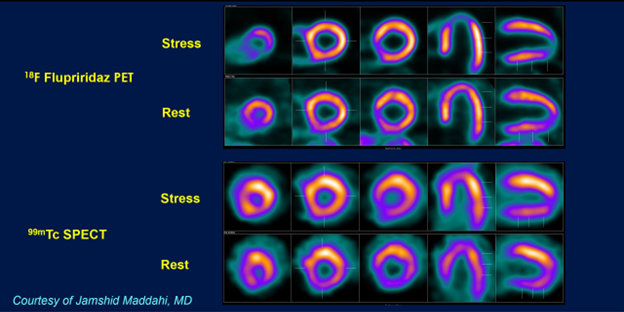
Representative rest and stress 18F Flurpiridaz PET and 99mTc SPECT myocardial perfusion images in a patient with 79% stenosis in the distal left anterior descending coronary artery. The 18F Flurpiridaz PET images have a higher resolution than the 99mTc SPECT images. Furthermore, 18F Flurpiridaz PET images show a definite perfusion defect in the apex that corresponds to the patient’s distal left anterior descending coronary artery stenosis. The 99mTc SPECT study do not show an apical defect and is therefore falsely negative for the presence of coronary artery disease.
“Flurpiridaz also is supposed to have a more linear relationship between flow and tracer uptake, which could improve the ability to perform quantification of perfusion,” he noted. “It also offers the ability to do exercise PET, which is impossible for rubidium, and challenging for ammonia, given its 11-minute half-life.”
Flurpiridaz Status
The FDA requires two phase-3 studies that show safety and sufficient diagnostic performance before it will approve a new tracer. The first required study, published in 2020 in the Journal of the American College of Cardiology, showed that the tracer’s sensitivity for detection of ≥50% stenosis by ICA was significantly higher than SPECT; however, the specificity did not meet the prespecified noninferiority criterion.
The second FDA-required study, published online recently, also in the Journal of the American College of Cardiology, was designed differently from the first in that only patients with suspected ― not known ― CAD were enrolled. The primary efficacy endpoint was sensitivity and specificity of flurpiridaz PET for overall detection of CAD, rather than comparing it to SPECT MPI (which became a secondary endpoint). PET and SPECT studies were both performed before invasive coronary angiography to minimize referral bias; SPECT studies included cadmium zinc telluride cameras.
In that study, which included 578 patients (mean age, 64; 32.5% women) from 48 centers in the US, Canada, and Europe, flurpiridaz met the efficacy endpoints: its sensitivity and specificity were significantly higher than the prespecified threshold value by two of the three readers; its sensitivity was higher than SPECT (80.3% vs 68.7%), and its specificity was noninferior (63.8% vs 61.7%).
PET areas under the receiver-operating characteristic curves were higher than SPECT in the overall population, women, and obese patients, at half the radiation dose of SPECT.
“Cardiac PET MPI is positioned to serve as the leading modality for the functional evaluation of suspected and known CAD,” Jamieson M. Bourque, MD, MHS, medical director of nuclear cardiology, echocardiography, and the Stress Laboratory, University of Virginia, Charlottesville, wrote in an editorial accompanying the second study. “18F-flurpiridaz will facilitate this upward progression with beneficial tracer characteristics that will increase access and availability, enable exercise stress, and optimize MBF quantification.”
At this point, FDA approval of flurpiridaz is expected sometime in 2024, said James E. Udelson, MD, principal investigator of the recent study, chief of the Division of Cardiology, and director of the Nuclear Cardiology Laboratory at Tufts University School of Medicine, Boston.
Learning Curve
Flurpiridaz comes with “a really interesting and important” learning curve, Udelson said. “The images are really crisp, and they look very different from what most people are used to. The GE folks are going to have to make sure that the American Society of Nuclear Cardiology and other professional societies are tuned in to help in the education part, because it’s not an easy, automatic switch. Very good image readers can adapt, but it’s not just one day you do one, then switch to the other.”
A “somewhat apt” analogy would be the difference between an echocardiogram and an MRI, he explained. “The MRI is much crisper. You’re seeing edges more crisply. You’re seeing the difference between a thicker and a thinner segment of the wall more crisply, and that’s actually real. You can’t say the thinner segment is abnormal; it’s just that you’re seeing it better. So, with this tracer, normal differences in the thickness of a wall can almost look like a defect if you’re not used to knowing that’s the new normal.”
The expected approval of flurpiridaz “will be a win for cardiac PET, broadening the range of sites that could perform PET,” Salerno commented. “However, it is worth cautioning that all of the prior data with PET using different agents does not necessarily equate to the same performance with the new agent, given that the performance seems to be lower than that shown in prior PET studies using other agents.”
Salerno would like to see additional studies comparing flurpiridaz with rubidium or ammonia, and also studies performing quantification with flurpiridaz, “which theoretically should have some advantages,” he said.
Udelson noted that MedTrace, a company in Denmark, is working on a radiolabeled water tracer based on 15-O-water that is just starting a pivotal trial. Udelson is a consultant to the company and is a steering committee member for the pivotal trial.
For now, “the big take-home is that there are a lot of ways these days to test people for CAD,” he said. “As the types of things we can do to test people expand, individuals and centers need to make sure they focus on providing any new service, however they do it, with really superb quality and experience.
“You don’t just do something new because it’s new,” he added. “It has to be done really well. If you do the new thing badly, you’re not going to get better information.”
Udelson is a consultant and advisory board member for GE Healthcare, a consultant to MedTrace, and a steering committee member for MedTrace’s pivotal trial. Bourque has served on a GE Healthcare advisory board for amyloid imaging. Salerno reports no relevant financial relationships.
Follow Marilynn Larkin on X: @MarilynnL.
For more from the heart.org | Medscape Cardiology, follow us on X and Facebook.
Source: Read Full Article