Hepatitis C and hepatitis B viruses both attack the liver, eventually causing deadly cirrhosis or cancer. But while antivirals can cure 95% of HCV infections, its cousin HBV has long eluded effective therapeutics. As a result, nearly 1 million people die from HBV every year.
Now researchers from the lab of Rockefeller’s Charles M. Rice—who shared the 2020 Nobel Prize in Physiology or Medicine for pioneering novel methods to grow and study HCV—have developed an approach for studying HBV in the lab that sidesteps the virus’s typical replication process, allowing for a much sharper view of its behaviors and characteristics during a crucial part of its life cycle. For too long, this view has been obscured, preventing researchers from improving drug treatments, let alone finding a cure.
Just as the Rice lab’s work on HCV exposed that virus’s weaknesses, the hope is that this novel approach could do the same for HBV.
“It’s important to study the whole virus life cycle, because every step is important for how the virus spreads and infects new cells,” says Bill Schneider, a research associate in Rice’s Laboratory of Virology and Infectious Disease, and a co-author on a paper in Science Advances describing their results. “Any one of those steps can potentially be exploited for vulnerabilities.”
Notoriously difficult to study
Despite sharing an affinity for the liver, HBV and HCV are quite different. They’re from different families, have different genetic compositions—HBV is a DNA virus, while HCV is an RNA virus—and HBV is only about one-third the size of HCV, with a unique genetic architecture that makes it more challenging to study.
But they do share some characteristics. They’re both easily transmittable and hard to shake once an infection has taken root. Some 296 million people live with a chronic HBV infection that is often asymptomatic until liver disease has advanced so far that it is largely untreatable.
Unlike with HCV, current HBV therapies can cause intolerable side effects or have limited impact, leading to lifelong drug therapy. And while there’s an effective vaccine that can block new HBV infections, it’s helpless against existing ones.
Progress has been slow for a few reasons. One is that HBV is notoriously difficult to culture in the lab. “It’s not clear why, because it’s an extremely effective virus in nature,” Schneider says. With origins dating to 400 million years ago, HBV and its relatives are capable of infecting a variety of animals.
Another is that methods commonly used to study HBV in the lab are plagued by background noise from HBV DNA and plasmids. The result is a lot of genetic fuzziness that makes it hard to clearly see the virus’s properties. “There’s just so much noise from the plasmids that it is difficult to distinguish something made from the replicating virus as opposed to the plasmid,” says first author Yingpu Yu, a research associate in the Rice lab. “It’s like throwing a lit candle into a bonfire and then trying to study the candle flame.”
Scientists have tried workarounds to tackle this problem, but Rice hit on a winning idea: Spark the virus’s life cycle later in the process using RNA, which might allow them to avoid all the DNA noise.
On the road to a potential cure
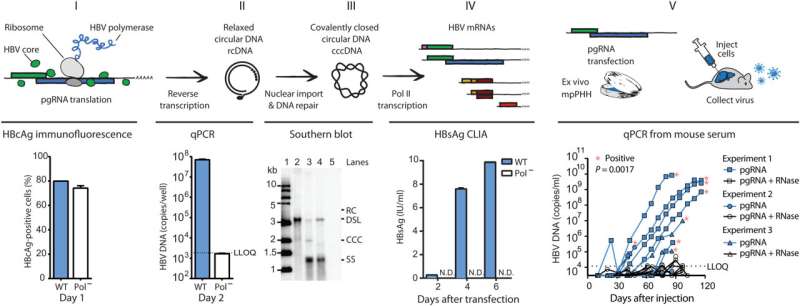
Like other viruses, HBV hijacks a cell’s molecular machinery in order to reproduce, but its process is a bit unusual. Once inside the nucleus, it uses that machinery to first transcribe its DNA to RNA, and then converts that into a new viral genome called covalently closed circular DNA (cccDNA). It’s these cccDNA genomes that are incredibly difficult to get rid of.
If they could start HBV’s life cycle before it produces cccDNA, that might give them an unobstructed view of the process at the heart of HBV’s ability to become a chronic, destructive tenant of the liver.
Thanks to HBV’s tiny size, it has few RNA transcripts involved in the replication process. One turned out to be key: pre-genomic RNA. Previous research had shown that pgRNA was able to instigate replication in a relative of HBV that infects ducks. They wondered if they could use it to spark the replication cycle in human HBV too.
It worked. First via cultured cells and then in mouse models, they were able to kickstart production of the virus’s cccDNA using its RNA, thereby silencing the background noise.
As they continued to exploit this strategy, they used a computational method called deep mutational scanning to look for HBV mutations that confer resistance to antivirals. They’ve already found several, some of which have been detected in infected patients, but never before in deliberately grown cell cultures, says Yu.
The new platform has great potential to play a role in the development of new therapies or a cure for HBV—just as Rice’s method did with HCV. Still, much more research is needed.
“Anywhere you can impinge on that life cycle and prevent this virus from replicating and spreading to new cells could be a potential target for new drugs,” Schneider says. “It’s not clear yet what the right combination of therapies will look like. All we know at the moment is that the ones that we have aren’t doing the job.”
More information:
Yingpu Yu et al, An RNA-based system to study hepatitis B virus replication and evaluate antivirals, Science Advances (2023). DOI: 10.1126/sciadv.adg6265
Journal information:
Science Advances
Source: Read Full Article