The significant mortality rate associated with the ongoing Coronavirus disease 2019 (COVID-19) pandemic has been linked to acute respiratory distress syndrome (ARDS) and multi-organ dysfunction. Mechanisms underlying the phenomenon remain unclear.
A new preprint research paper posted to the medRxiv* server points to mast cells as major culprits in the etiology of severe and critical COVID-19. This could suggest suitable therapeutic and preventive interventions to reduce the morbidity and mortality of this viral illness.
Background
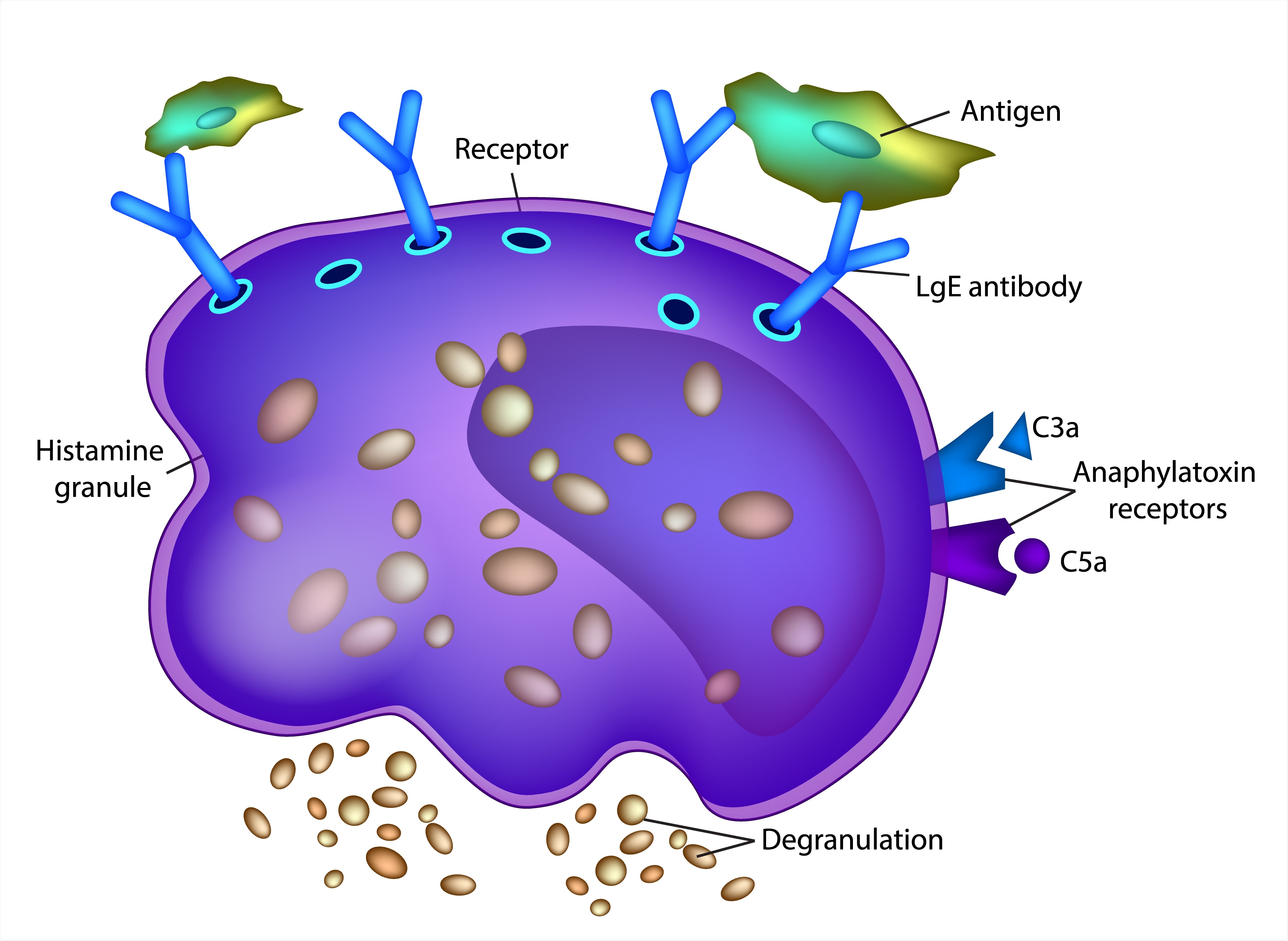
Mast cells are granulocytes, white blood cells with granules containing a number of potent chemicals such as histamine, serotonin, and protein-digesting enzymes such as chymase and tryptase. They also contain cytokines and lipid mediators, common to all granulocytes.
These cells have a long lifespan and are present in both connective tissue and mucous membranes. In adult life, they are found in mature form only within the tissues, while in the blood, only their immature precursors are found, forming less than 0.005% of the total blood cells. Tissue-resident mast cells express multiple pathogen recognition receptors on the surface and within the cytoplasm.
Mast cell degranulation produces inflammation, recruiting other immune cells such as monocytes and neutrophils, and T lymphocytes.
These cells also affect the vascular tone and permeability through the vasoactive contents of their granules. By so doing, they may induce tissue hypoxia via shunts, bypassing the capillary bed. This may result in damage to both the tissue and the vascular structure.
Mast cells have diverse phenotypes depending on the tissue of residence. Those in the lung of individuals with atopy express Immunoglobulin E (IgE) receptor, FcεR1 at higher levels relative to the skin, and are implicated in the severe inflammation of the lung that occurs in asthma.
Mast cells bring together immune responses against viruses and other pathogens, but they may be harmful to the host as well, as shown by the above observations. In influenza, for instance, mast cell stabilization led to better control of lung lesions.
When mast cell activation remains at a high level systemically, the vascular and coagulation systems are affected. This is mediated by chymase and other granule enzymes. This is seen, for instance, in dengue hemorrhagic fever, with leaking blood vessels and clotting defects.
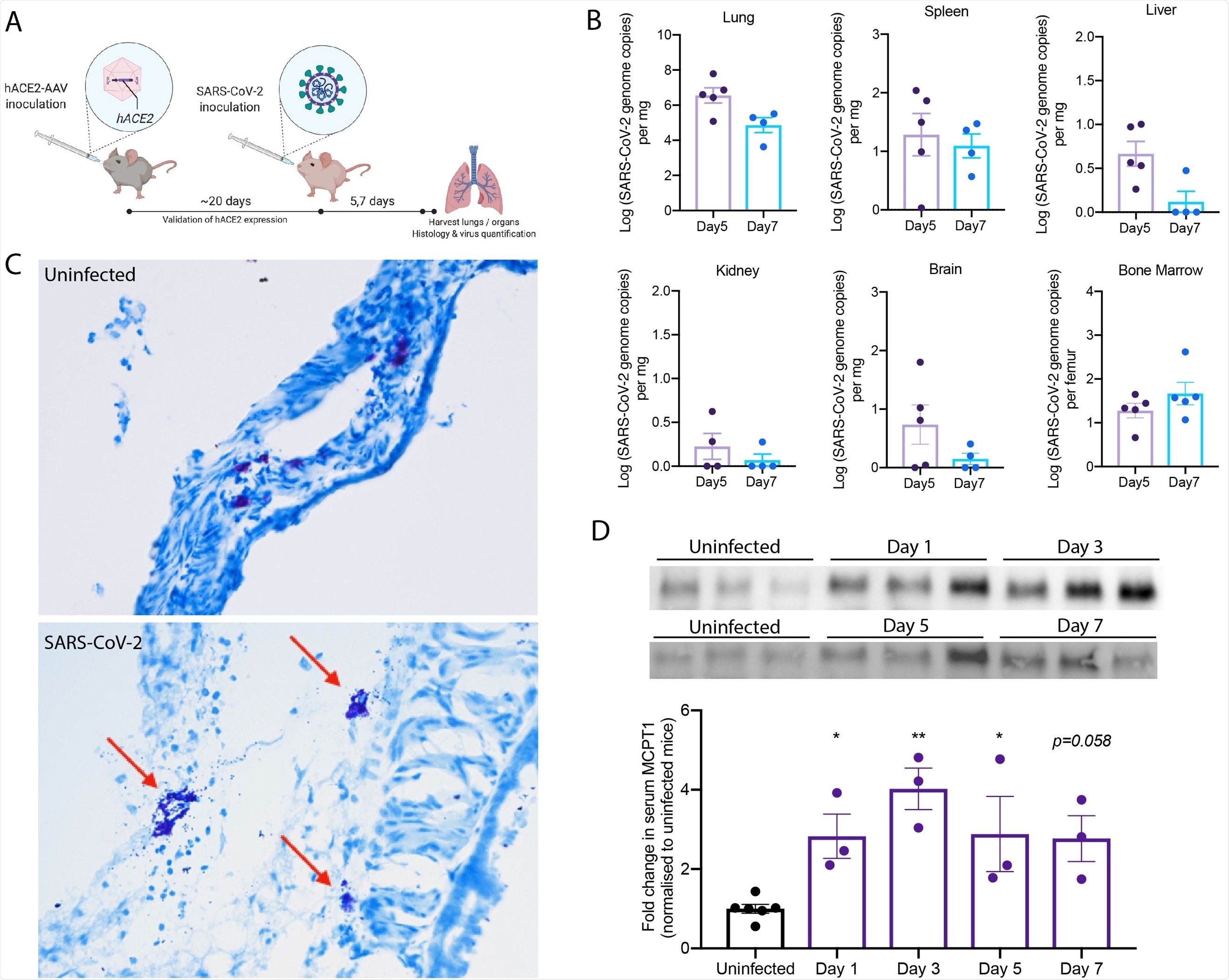
The current study adds to this knowledge by showing that mast cell degranulation is widespread in the lung tissue in both acute and recovering mice and non-human primate (NHP) animal models infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and in human COVID-19 patients.
Animal studies confirm mast cell activation
In mice, mast cell degranulation was found in the airways, while resting mast cells were found in the healthy trachea. Tracheal inflammation and swelling were also found in infected animals.
Mouse chymase levels were high, this being a biomarker of mast cell activation, beginning to decrease with the reduction of the viral burden.
Genes relating to mast cell precursors found in peripheral blood were highly expressed in patients with severe COVID-19. This was accompanied by the upregulation of many host response pathways for the main mast cell products.
Chymase from mast cells was present at high levels in the sera of these patients, further proof of mast cell activation in COVID-19. Thus, SARS-CoV-2 causes mast cell activation in vivo.
In NHPs, severe lung disease was observed in all animals, with areas of bleeding and fluid build-up. Necrotic patches were present on the lungs in half the cases. Degranulated mast cells were seen in many places within lung tissue, as well as free granules outside the mast cells.
The greatest concentration of mast cells was seen within the areas of hemorrhage. These findings indicate that SARS-CoV-2 infection causes lung lesions with persistent mast cell activation at 21 days after acute infection.
Since mature mast cells are not found in blood, their transcriptional profile was explored to detect systemic elevation of mast cell products. This showed that mast cell-associated gene expression increased with severe disease, some in the acute phase and some when the illness was resolving.
These changes were not seen to evolve over time in mild COVID-19, though individual gene changes did occur. Overall, severe COVID-19 results in the activation of key immune receptors on mast cells, as shown by disrupted downstream pathways. Mast cells are therefore likely to be important in causing severe disease.
Human studies show mast cell-mediated damage
In human patients with severe COVID-19, mast cell maturation markers were increased. Epithelial junction signaling was activated in response to mast cell proteases to increase vascular permeability. Similar was the case with pro-inflammatory pathways that are responsive to mast cell products.
The renin-angiotensin pathway was also perturbed, perhaps due to the activity of mast cell chymase which is responsible for angiotensin II production outside of ACE (angiotensin-converting enzyme) activity. In fact, chymase levels in hospitalized COVID-19 patients were markedly higher than in those with mild disease.
Notably, chymase-mediated angiotensin II is found in atherosclerosis of the aorta and in early lung vascular disease, and some evidence suggests its implication in lung injury in COVID-19. Angiotensin II is elevated, pushing up the angiotensin I/angiotensin II ratio in severe COVID-19.
The difference was most marked when intubated patients were considered vs. outpatients with COVID-19. The former also showed elevated markers of endothelial activation, which is associated with severe COVID-19 – again, as expected following mast cell activation. Thus, both high chymase levels and mast cell activation are characteristic of severe COVID-19.
What are the implications?
Mast cell activation is thus likely to increase the intensity of inflammation, delaying recovery and enhancing tissue injury, as suggested by the findings in mouse models. The NHP experiments indicate persistent inflammatory damage even after the infection subsides because of the antibody response.
Mast cells may be activated in the resolving phase of infection by autoantibodies since they have to activate FcγRs on their surface for antigen- IgG antibody immune complexes. This may partly account for long COVID-19 but must be established by more research.
It is possible that mast cell precursors in the blood are recruited and are responsible for the increased transcription of pro-inflammatory chemicals observed in this study. This is more likely since elevated chymase transcription was not identified, it being a specific product of mature mast cells.
These precursors may migrate into the lung since the chemokine CXCR2 is also raised in patients with severe COVID-19. Tissue-resident mast cells have a sentinel function, detecting allergens and infectious pathogens and recruiting many immune cell types into the inflamed tissues.
Of course, the coagulation and vascular events in some severe COVID-19 patients are also explained by mast cell activation, as these cells line the blood vessels within tissues. Their degranulation affects the lung vascular bed, causing endothelial damage and disrupting pulmonary blood flow and oxygenation, resulting in tissue damage.
A similar phenomenon is seen with dengue, where the virus does not directly infect the lungs but activates endothelial cells, causing microvascular leakage and hemorrhage. This is enhanced by mast cells, which help clear the virus in early dengue, but paradoxically worsen disease severity in the later stages.
“Drugs targeting MCs and their products are promising as a therapeutic strategy to prevent severe clinical courses in DENV infection and may bear similar promise in preventing severe COVID-19, which warrants further evaluation.”
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Tan, J. et al. (2021). Signatures of mast cell activation are associated with severe COVID-19. medRxiv preprint. doi: https://doi.org/10.1101/2021.05.31.21255594. https://www.medrxiv.org/content/10.1101/2021.05.31.21255594v1
Posted in: Medical Research News | Disease/Infection News
Tags: Acute Respiratory Distress Syndrome, Angiotensin, Antibody, Antigen, Aorta, Asthma, Atherosclerosis, Autoantibodies, Biomarker, Bleeding, Blood, Blood Vessels, Bone, Bone Marrow, Brain, Cell, Chemicals, Chemokine, Coronavirus, Coronavirus Disease COVID-19, Cytokines, Cytoplasm, Drugs, Edema, Enzyme, Fever, Gene, Gene Expression, Genes, Hemorrhagic Fever, Histamine, Histology, Hypoxia, Immunoglobulin, in vivo, Inflammation, Influenza, Kidney, Liver, Lung Disease, Lungs, Mast Cell, Mortality, Neutrophils, Pandemic, Pathogen, Protein, Receptor, Renin, Research, Respiratory, SARS, SARS-CoV-2, Serotonin, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Skin, Spleen, Syndrome, Transcription, Vascular, Virus, Western Blot

Written by
Dr. Liji Thomas
Dr. Liji Thomas is an OB-GYN, who graduated from the Government Medical College, University of Calicut, Kerala, in 2001. Liji practiced as a full-time consultant in obstetrics/gynecology in a private hospital for a few years following her graduation. She has counseled hundreds of patients facing issues from pregnancy-related problems and infertility, and has been in charge of over 2,000 deliveries, striving always to achieve a normal delivery rather than operative.
Source: Read Full Article