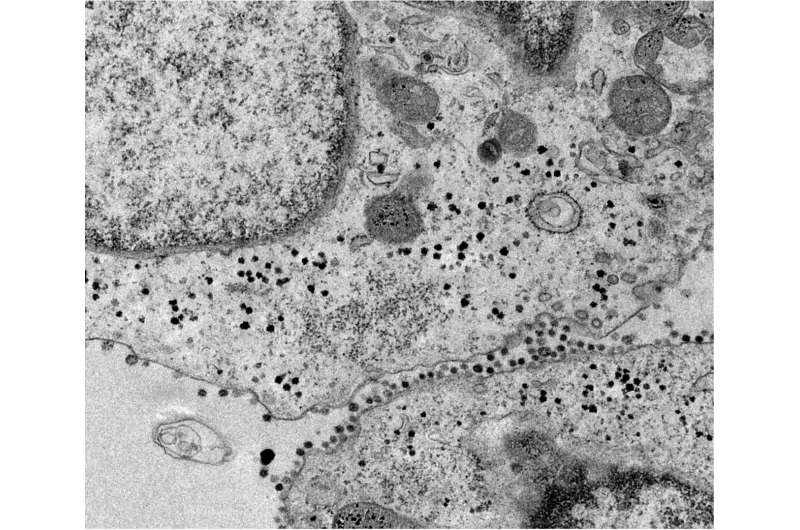
For two years, thousands of scientists and doctors around the world have been working to understand how COVID-19 develops and what relationship it has with other types of diseases. Various studies indicate that people with diabetes are more likely to develop severe COVID-19, as well as that more than 20% of patients hospitalized for COVID-19 suffer acute kidney damage. However, to date, it was unknown what was the factor that caused this to happen.
Now, an international team led by Nuria Montserrat, ICREA research professor at the Institute of Bioengineering of Catalonia (IBEC) and international collaborators have used bioengineering to develop mini-kidneys that simulate the kidney of a person in the early stages of diabetes.
In this international collaboration the researchers have provided, for the first time, the use of kidney organoids to understand the early stages of diabetes in this organ. In order to demonstrate that the ACE2 receptor plays an essential role in SARS-CoV-2 infection in the kidney, the team has also used genetic engineering to generate defective organoids for other receptors described to date as gateways for the virus. Using patient kidney cells, this study also reveals the role of energy metabolism in SARS-CoV-2 infection, opening the door to the identification of new therapeutic interventions to treat COVID-19. This groundbreaking study has just been published in the journal Cell Metabolism.
Diabetic mini-kidneys have more portals of entry for SARS-CoV-2
Mini-kidneys have been developed in the laboratory from pluripotent human stem cells. To reproduce the diabetic environment, the researchers have subjected the mini-kidneys to culture conditions that result in the generation of mini-kidneys with the same cellular characteristics and metabolic alterations as those found in the kidneys of a person with early-stage diabetes.
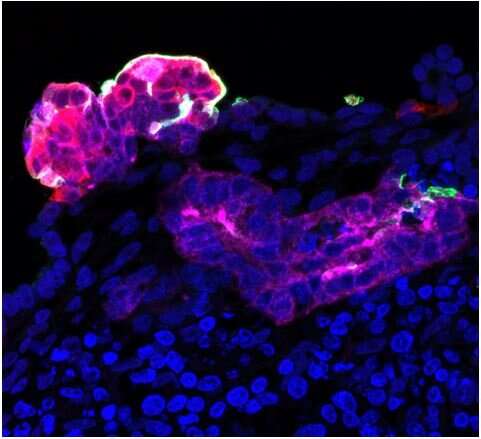
Using different molecular biology techniques, such as gene editing, the researchers have observed that, in diabetic mini-kidneys, it is the abundance of the ACE2 receptor that determines susceptibility to viral infection, establishing a causal relationship between diabetes and the presence of one of the receptors described so far as determinant in SARS-CoV-2 infection.
“Our diabetic renal organoid model has allowed us to observe that diabetic mini-kidneys, with a greater number of ACE2 receptors, have a greater susceptibility to viral infection,” says Elena Garreta, Institute for Bioengineering of Catalonia and first co-author of the study.
“It is absolutely imperative to understand the molecular mechanisms that underlie more severe COVID-19 in patients with diabetes and other metabolic comorbidities. The development of a diabetic kidney organoid is a great step towards experimentally dissecting how metabolic changes can impact SARS-CoV-2 infections. The data again demonstrate that ACE2 is the essential receptor for SARS-CoV-2 even under conditions of comorbidity,” says Josef Penninger, Institute of Molecular Biotechnology.
Furthermore, using state-of-the-art techniques such as RNA sequencing, the researchers identified that diabetic mini-kidneys have a metabolic signature that could explain why diabetic mini-kidneys become more infected.
Diabetes increases susceptibility to SARS-CoV-2 infection in patient cells.
To verify whether the results obtained with the mini-kidneys are also observed in the native organ, the researchers analyzed kidney cells from patients with diabetes and individuals without diabetes. The data show that kidney cells from diabetic patients, in the same way as what happens in mini-kidneys, have more ACE2 receptors and suffer a higher rate of SARS-CoV-2 infection. To delve into the mechanisms that may explain such observations, the researchers used a compound that modulates the metabolic state of cells and found that the treatment reduced viral infection.
“This finding sheds a light on a potential mechanism behind more severe cases of diabetic patients. This technology will improve our capability to investigate how the virus interacts with different organs in the human body,” says Ali Mirazimi, adjunct professor at Karolinska Institutet and one of the study’s corresponding authors.
Source: Read Full Article