The clean-up of cellular “protein clumps” could prevent the onset of some types of dementia, according to a new study from The University of Queensland.
Researchers from the Queensland Brain Institute made the discovery after focusing on the relationship between the enzyme Fyn and the protein Tau in frontotemporal dementia. The team, led by Professor Frederic Meunier and Dr. Ramón Martínez-Mármol, found that Fyn, an important player in learning and memory, became highly active when it is immobilized within the synapses which are the connection hubs between neurons where neuronal communication takes place.
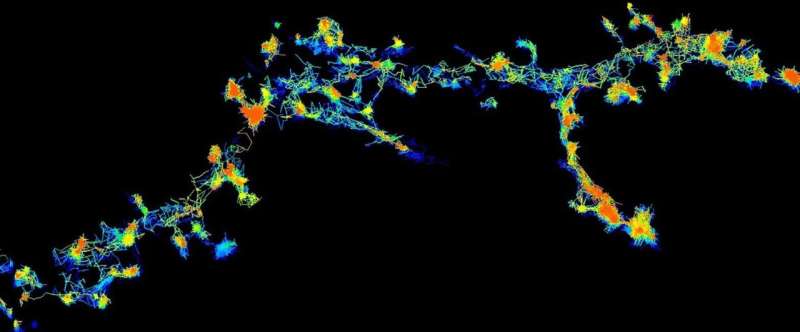
“Using super-resolution microscopy, we can now see these enzymes individually and in real-time, moving around randomly in live neurons,” Lead author Dr. Martínez-Mármol said.
The team found that when these enzymes become activated, they change to an opened structure (like a flower that blossoms) and slow down their movement, grouping together to form clusters or clumps of proteins, before refolding and dispersing to start their cycle again.
“When they need to complete an action, the Fyn enzymes slow down and congregate at the synapse to initiate their function,” Dr. Martínez-Mármol said.
Normally, this process occurs naturally thousands of times at the synapses between neurons and is necessary to maintain neuronal communication, which is the basis of learning and memory.
Professor Frederic Meunier explained that for learning and memory to occur, Fyn needs to form these dynamic clusters. “But if you alter the balance in any way—you have too little, or too much clustering, you develop pathological issues,” he said.
https://youtube.com/watch?v=OQzuxf1XIYg%3Fcolor%3Dwhite
The research follows the team’s earlier work, where they discovered Tau impacted a critical mechanism in memory function. The team showed, using super-resolution microscopy, that when neurons are exposed to a mutant version of Tau present in frontotemporal dementia, the clustering of Fyn enzyme is accentuated with the potential to trigger a debilitating chain reaction.
The association of Fyn and Tau necessary for the progression of different forms of dementia, including Alzheimer’s disease and frontotemporal dementia, has been demonstrated by many laboratories around the world; however, the precise molecular mechanisms behind this pathological interaction were not known.
Importantly, this mutant Tau has a higher propensity to form what is known as biomolecular condensates, which are small gel-like droplets within the cells. Some proteins, under specific conditions, tend to spontaneously aggregate, forming droplets that resemble oil spills in an aqueous solution. Tau is one of these proteins.
If formed at the neuronal synapses, these Tau droplets create the perfect trap for Fyn molecules, keeping them highly immobile and accentuating their clustering and activation for longer.
“It’s like a spider web,” Dr. Martínez-Mármol said. “Normally, Fyn stops and moves, stops and moves. In frontotemporal dementia, Fyn stops more as it becomes stuck in this gel-like structure. The droplets of Tau, therefore, attract additional Fyn proteins at the synapse.”
Professor Meunier said Tau biomolecular condensates could hold the key to reverting this toxic chain reaction.
“We believe they are the perfect target for future therapy to re-establish normal Fyn clustering dynamics,” Professor Meunier said. “Theoretically, attacking the formation of toxic Tau biomolecular condensates should prevent the process of dementia from happening.”
The study has been published in Molecular Psychiatry.
More information:
Ramón Martínez-Mármol et al, Fyn nanoclustering requires switching to an open conformation and is enhanced by FTLD-Tau biomolecular condensates, Molecular Psychiatry (2022). DOI: 10.1038/s41380-022-01825-y
Journal information:
Molecular Psychiatry
Source: Read Full Article