In many human endeavors, having good tools for a particular task is an essential requirement to obtain the best results possible, and neuroscience is no different than other scientific fields in this regard. However, neuroscientists tackle the colossal objective of shedding light on the inner workings of neurons and neuronal circuits, and they rely on various methods to observe and control the firing of neurons to gain a better understanding of their functions.
Optogenetics has been regarded as one of the most impactful breakthroughs in neuroscience over the last decades. It involves using light of specific frequencies to control neurons in genetically modified organisms. Neurons, like all cells, have ‘ion channels’: membrane proteins that can be opened or closed and regulate the flow of charged particles in and out of the cell, thus regulating the electrical behavior of neurons. Now, thanks to optogenetics, they can be altered to be light-sensitive—light essentially can be used to open or close these channels in genetically modified organisms, giving researchers control over which and when neurons fire. Now, a new study by scientists from Okayama, Japan, proposes a promising new tool based on optogenetics for studying neurons. But why is this tool so attractive to neuroscientists?
While optogenetics has certainly facilitated our understanding of neurons, the technique has some limitations. In particular, the available light-sensitive variants of negatively charged ion, or ‘anion,’ channel proteins, which regulate the flow of negatively charged ions, are much less diverse than their positively charged ion, or “cation” channel counterparts (for positively charged ions). Whereas light-sensitive cation channels can be used to ‘activate’ neurons using light, light-sensitive anion channels act as neuron ‘silencers’ that prevent the neuron from firing when illuminated at the right frequency.
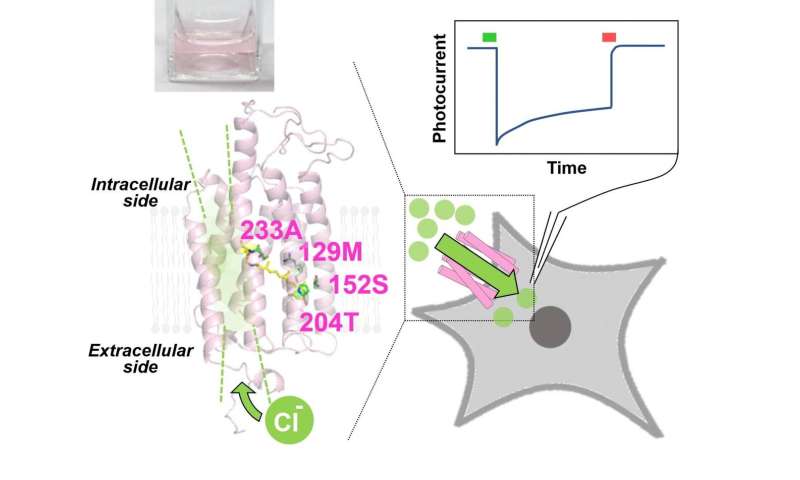
This new study by Japanese scientists, published in The Journal of Physical Chemistry Letters, expands the available options for optogenetic neuronal silencing, and explores the potential of GtACR2, a natural light-regulated anion channel from an alga.
The scientists first introduced strategic mutations in GtACR2 amino acids to produce more light-responsive anion channels and tested the results using Escherichia coli bacteria. They found a shift in the frequency that was required to open the channel. The mutant GtACR2 channels also remained open much longer than their normal counterparts. Dr. Yuki Sudo, Dr. Keiichi Kojima and Ms Natsuki Miyoshi of Okayama University, who led the study, remarks: “Long-time neural inhibition generally requires repetitive long-lasting illumination; however, this invariably heats tissues, causing physiological and behavioral changes and tissue damage. Using the observed prolonged channel opening, GtACR2 mutants would be an effective neural silencer over long-term scales with lower illumination time and fewer heat-dependent effects.”
GtACR2 mutants can also be activated and inactivated by illuminating them with different light frequencies. Irradiating the channels with green light opens them, silencing the neuron, but irradiation with red light causes them to quickly close. This ‘step-functional’ property could give future scientists finer control over the state of the channels and the associated neurons, providing a more sophisticated tool for neurological experiments.
Source: Read Full Article