Like other countries in the world, Japan has witnessed a worrisome increase in the prevalence of chronic rhinosinusitis (CRS) over the last decade. An inflammatory disease that lasts at least 12 weeks, CRS can cause nasal congestion, nasal discharge, trouble breathing through the nose, facial pain, and even loss of sense of smell.
Unfortunately, treating CRS is complex since the disease manifests in various forms. CRS can be categorized into eosinophilic (ECRS) or non-eosinophilic (non-ECRS) types. In ECRS, the nasal and sinus tissues exhibit an increased presence of eosinophils, a type of white blood cell that releases inflammatory compounds.
The increased prevalence of CRS is largely driven by environmental factors that are in turn impacted by lifestyle changes. Of the several environmental factors, microorganisms residing in the nasal cavity and passages have been known to substantially affect our health. It is however, unclear whether the nasal microbiome contributes to the development of ECRS.
To address this knowledge gap, a research team from Japan led by Assistant Professor Masanori Kidoguchi from the Faculty of Medical Science of the University of Fukui, Japan, recently conducted a study on CRS in a Japanese population with a focus on the nasal microbiome.
Their paper, which was also co-authored by Professor Shigeharu Fujieda from the University of Fukui and Professor Emiko Noguchi from the University of Tsukuba, was published in The Journal of Allergy and Clinical Immunology on September 25, 2023. Dr. Kidoguchi said, “We undertook this study because the pathological functions of bacteria and their metabolites in the development of ECRS remain unknown.”
First, the researchers collected nasal swabs from 143 subjects, of which 65 had ECRS, 45 had non-ECRS, and 33 were healthy control subjects. They then compared the microbiome diversity between the CRS and control groups from these samples and found significant differences, suggesting that the nasal microbiome is indeed involved in (or affected by) the disease.
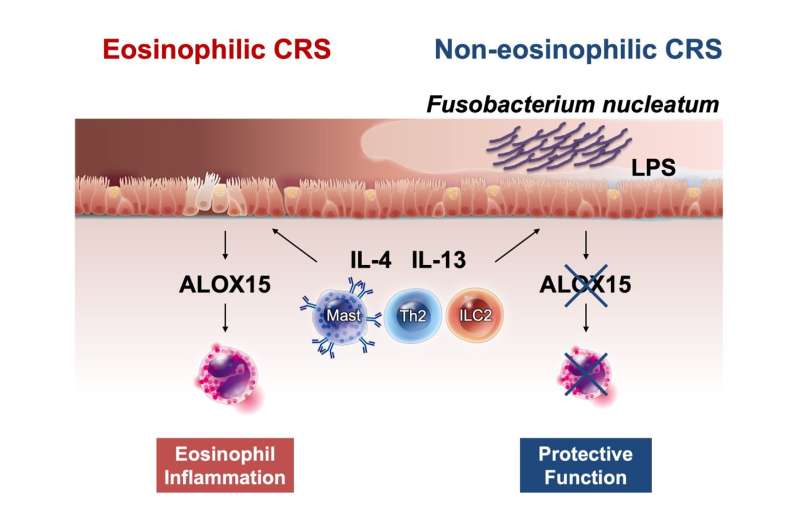
More importantly, the microbiome composition differed significantly between the ECRS and non-ECRS groups. Through chemical and genetic testing, the team found that the bacterium Fusobacterium nucleatum (F. nucleatum) was less abundant in patients with ECRS. Furthermore, metagenomic analyses revealed that lipopolysaccharide (LPS) synthesis was greater in patients with non-ECRS than in those with ECRS.
Based on these results, Dr. Kidoguchi said, “F. nucleatum is known to cause inflammation by producing LPS. Some studies suggest that LPS has varying structures and functions depending on the bacterial species. We therefore hypothesized that LPS derived from F. nucleatum might be linked to the pathogenesis of both ECRS and non-ECRS.”
To test this hypothesis, the team investigated whether LPS isolated from F. nucleatum had an effect on the expression of specific cytokines in human bronchial epithelial cell cultures. Their experiments showed that LPS derived specifically from F. nucleatum suppressed the expression of ALOX15, an enzyme that plays a key role in the formation of nasal polyps and eosinophil-related inflammation.
Taken together, the results of this study reveal that disruptions in the nasal microbiome likely play a critical role in ECRS. This finding could be leveraged to develop more effective strategies to combat this troublesome condition.
“The microbiome may strongly influence treatment resistance in CRS and may have an impact on other allergic diseases as well,” comments Dr. Kidoguchi. “Future studies will hopefully lead to probiotic development and lifestyle modification methods for preventing refractory chronic sinusitis.”
More information:
Masanori Kidoguchi et al, Middle meatus microbiome in patients with eosinophilic chronic rhinosinusitis in a Japanese population, Journal of Allergy and Clinical Immunology (2023). DOI: 10.1016/j.jaci.2023.06.029
Journal information:
Journal of Allergy and Clinical Immunology
Source: Read Full Article