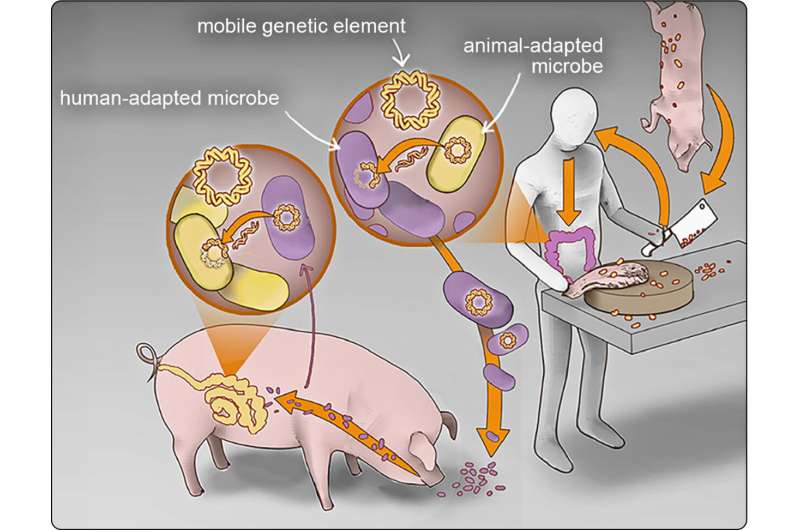
A new study suggests that Escherichia coli and other disease-causing microbes are passing easily between humans and animals in Cambodia, a country where clean water, sanitation and hygienic controls are lacking in many regions. The continuous exchange, along with unregulated antibiotic use, leads to the emergence and spread of drug-resistant E. coli, the authors say.
Maya Nadimpalli, a scientific collaborator at the Antibiotic Research Action Center at the George Washington University and her international colleagues, conducted the research in Phnom Penh, an urban area where humans and animals are often living in close proximity without clean water or other environmental controls that help prevent the spread of E. coli and other potentially dangerous microbes.
Nadimpalli worked with Lance B. Price, the Director of the Antibiotic Resistance Action Center at GW, and his team, to compare the genetic sequence of E. coli obtained from humans living in Phnom Penh and meat bought in the marketplace there. The team identified multiple genetic elements that confer resistance to powerful antibiotics that were widely dispersed among humans and food production animals in Phnom Penh.
“The results from our study were jaw dropping. They suggested that E. coli strains from people and animals were exchanging DNA at rates that we don’t see in high-income countries. And the DNA that they were sharing makes them resistant to some of the most important antibiotics in human medicine,” said Price, who also is a professor of environmental and occupational health at the George Washington University.
“Countries that lack widespread access to clean water and other environmental controls could be inadvertently brewing the next pandemic.”
The study suggests that the environment in Phnom Penh created many opportunities for the exchange of bacteria and genetic elements that confer resistance to antibiotics. Most meat and fish sold in Phnom Penh markets are grown in rural areas. A lack of sewage treatment in rural settings can result in the contamination of drinking water for humans and animals.
Farmed fish can be fed waste from pigs and poultry. In addition, some people in Phnom Penh routinely consume undercooked meat or fish, noted Nadimpalli, who is also an assistant professor of environmental health at Emory University Rollins School of Public Health.
“Ensuring consistent access to clean water and sanitation can improve people’s health and well-being in so many ways,” Nadimpalli said. “We’ve suspected that it could also help antibiotic resistance, but our findings show that improving access to clean water and sanitation is actually required—both in humans and food animals—if we want to have a fighting chance at preserving antibiotics for human health.”
The study showed that strains of E. coli in Phnom Penh had developed resistance to two powerful antibiotics used to treat humans–cephalosporins and fluoroquinolones. People who are sickened by eating food or being exposed to drug-resistant E. coli and other microbes can develop life-threatening infections. Public health experts estimate that antibiotic resistant bacterial infections cause more than 1.3 million deaths worldwide each year.
In high-income countries like the United States, experts focus on developing more effective antibiotics and getting people to avoid unnecessary antibiotic use.
However, this study suggests that the problem of antibiotic resistance will not go away unless public health officials address the lack of consistent access to basic sanitation and other environmental controls in countries where food animal production is intensifying.
“This is an urgent public health problem that will require policymakers, researchers and others to work together to reduce the risk,” Price said. “To combat this threat, experts will have to address the lack of basic sanitation and other environmental controls that are leading to the emergence and spread of antibiotic-resistant pathogens that threaten us all.”
The study, “Plugging the leaks: antibiotic resistance at human-animal interfaces in low-resource settings,” was published in Frontiers in Ecology and the Environment.
More information:
Maya L Nadimpalli et al, Plugging the leaks: antibiotic resistance at human–animal interfaces in low‐resource settings, Frontiers in Ecology and the Environment (2023). DOI: 10.1002/fee.2639
Journal information:
Frontiers in Ecology and the Environment
Source: Read Full Article