The lifespan of a small roundworm that has been used as a key model organism in aging research is limited by how it self-sacrifices to feed its young, finds a new study led by UCL researchers.
The authors of the new Nature Communications paper say their findings raise questions about how well insights from the Caenorhabditis elegans (C. elegans) worm can be translated to human aging advances.
C. elegans is widely used as a laboratory animal, and has been central to aging research for 40 years thanks to discoveries of genes that can be suppressed to produce up to a tenfold increase in the worm’s lifespan.
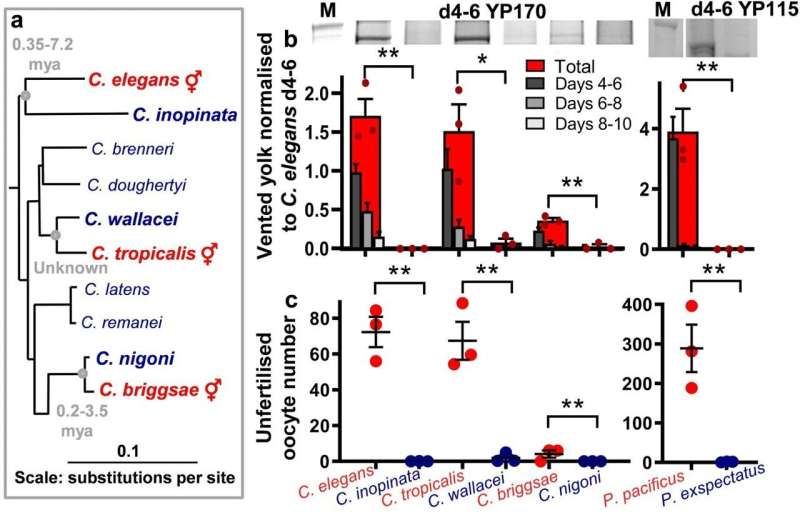
The UCL research team investigated what drives the lifespan of C. elegans, to see if the determining factors are markedly different from the aging processes of higher (larger, more complex) organisms. In a previous study, they had already identified an unusual reproductive trait in the worm, called yolk venting, which is a self-destructive process whereby the worm secretes a milk-like fluid through its vulva that is consumed by its offspring to support its grown.
By comparing the worm to other related Caenorhabditis species, the researchers have now found that yolk venting only occurs in Caenorhabditis species that are hermaphroditic (producing both male and female reproductive cells), while such species are also shorter-lived, as reproductive self-destructive processes were linked to shorter lifespan.
The researchers say their findings suggest that C. elegans is one of small number of animals known to have a single act of reproduction which is facilitated by self-destruction (another such species is the Pacific salmon, which swims upstream to spawn and dies soon after). Blocking reproduction, and so blocking self-destruction, is what leads to the huge extension of lifespan in such animals. Because of this, the aging mechanism in C. elegans is not directly comparable to that of humans.
Corresponding and co-lead author Professor David Gems (UCL Institute of Healthy Aging, UCL Biosciences) said, “This discovery is groundbreaking because it calls into question 40 years of orthodoxy in the field. The discovery suggests the dream of single gene mutations having a huge impact on human aging, as in the case of laboratory animals, is more fantasy than reality.”
Yet the researchers say there is still more to learn about human aging from C. elegans. In an article accompanying the study, the authors present evidence that suicidal reproduction has evolved from more general mechanisms of aging, that are applicable to humans. This can explain why targeting such mechanisms therapeutically can lead to more modest lifespan extensions in larger animals such as mice.
First and co-lead author Dr. Carina Kern (UCL Institute of Healthy Aging, UCL Biosciences) said, “While we can’t just switch off a troublesome gene to extend human life, we are now starting to understand a whole new suite of mechanisms that lie at the root of diseases of later life.”
“What we need critically to move the dial in the aging space is to understand the mechanisms that cause the process of aging in animal models and explain the causes of age-related disease more generally. We don’t yet understand this fully for any organism. But for C. elegans we are getting there, and the discovery of suicidal reproduction in the worm gets us another step closer.”
“Aging theory has also so far been obsessed with single causal explanations of aging, partially stemming from the discovery of these fountain of youth genes. Instead, what we need is an approach that is more holistic and accounts for the multiple biological mechanisms at play—a molecular pathway map, if you will.”
More information:
Carina C. Kern et al, C. elegans ageing is accelerated by a self-destructive reproductive programme, Nature Communications (2023). DOI: 10.1038/s41467-023-40088-1
Journal information:
Nature Communications
Source: Read Full Article