A new study of the aggressive brain cancer glioblastoma suggests that two specific cancer cell mutations may work together to help hide tumors from the immune system, offering a possible way to predict whether the tumors would respond to an emerging class of immunotherapy drugs. The research appears in Cell Reports.
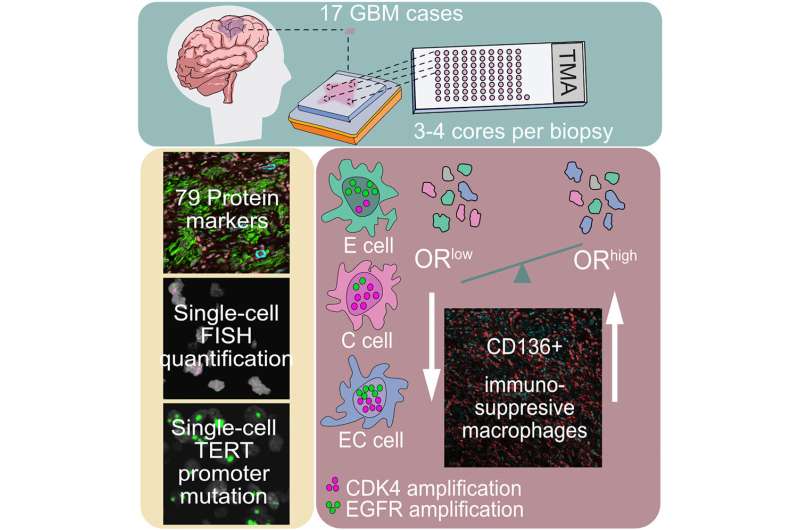
Glioblastoma samples from 17 patients’ tumors were analyzed by Michalina Janiszewska, Ph.D., a cancer biologist at The Herbert Wertheim UF Scripps Institute for Biomedical Innovation & Technology, in collaboration with Franziska Michor, Ph.D., a computational biologist with the Dana-Farber Cancer Institute in Boston.
The team combined statistical and computational tools with microscope techniques that highlight genetic mutations at the single-cell level. The data revealed a clear signal: If a tumor had a higher frequency of cells possessing more than six repeats each of two known cancer genes, EGFR and CDK4, it predicted the invasion of anti-inflammatory white blood cells known as macrophages into the tissue. The ones invading many brain tumor samples are known to suppress inflammation, which can hide cancer from immune attack, Janiszewska said.
A new class of drugs targets these macrophages, but early small studies have suggested they aren’t sufficiently effective for glioblastoma. Focusing on the subset of patients who would be more likely to benefit could give different results, she said.
“Our study suggests that using simple genetic testing and measuring tumor cellular diversity could, in the future, identify patients who would respond to therapies targeting these specialized pro-tumorigenic immune cells,” Janiszewska said.
Glioblastoma is a fast-moving cancer and the most common type of brain cancer. Once patients are diagnosed, their survival time is generally less than a year and a half, so new treatment options are needed. About 12,000 people a year in the United States are diagnosed with the disease.
The new class of cancer immunotherapies that attack macrophages are called CSF1R inhibitors. The first to be approved by the U.S. Food and Drug Administration was Turalio in 2019. What’s needed is an ability to predict which patients would be most likely to respond to such drugs, she said.
“There were trials of these therapies, but they looked like they didn’t provide benefit to the patients. It would be good to go back to the trial to see if we could tell the difference between the responders and the non-responders,” she added.
Janiszewska focuses on characterizing what she calls tumors’ “microenvironment.” This looks at the community of cells, tissue types, immune and other factors that influence a cancer’s growth and spread throughout the body. It’s a data-intensive new front in the war on cancer that blends computational biology, computer science and lab research to help develop more precise, individualized, effective cancer treatments.
From here, Janiszewska wants to collaborate with teams that have led the initial glioblastoma immunotherapy clinical trials to analyze tissue samples. She also wants to develop mouse models of that pair of mutations in glioblastoma, to learn more about the mechanism that leads them to draw in macrophages that cloak tumors.
“This study definitely allows us to see that there is a connection between the genetic diversity of an individual region of the tissue and the tumor microenvironment,” Janiszewska said.
More information:
Kacper A. Walentynowicz et al, Single-cell heterogeneity of EGFR and CDK4 co-amplification is linked to immune infiltration in glioblastoma, Cell Reports (2023). DOI: 10.1016/j.celrep.2023.112235
Journal information:
Cell Reports
Source: Read Full Article