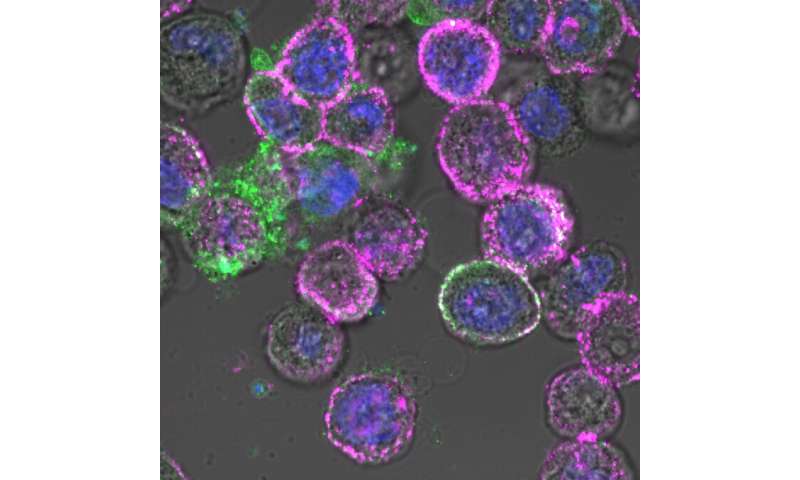
City of Hope scientists have combined two potent immunotherapies—an oncolytic virus and chimeric antigen receptor (CAR) T cell therapy—to target and eradicate solid tumors that are otherwise difficult to treat with CAR T therapy alone, according to a new Science Translational Medicine study.
In preclinical research that could lead to a clinical trial for patients with intractable solid tumors, City of Hope scientists genetically engineered an oncolytic virus to enter tumor cells and force their expression of CD19 protein on their cell surface. Scientists were then able to use CD19-directed CAR T cells to recognize and attack these solid tumors.
CD19-CAR T cell therapy is approved by the U.S. Food and Drug Administration to treat certain types of blood cancers, namely B cell lymphomas and acute lymphoblastic leukemia. This new research may expand the use of CD19-CAR T cells for the treatment of patients with potentially any solid tumor.
“Our research demonstrates that oncolytic viruses are a powerful and promising approach that can be combined strategically with CAR T cell therapy to more effectively target solid tumors” said Saul Priceman, Ph.D., the study’s senior author and an assistant professor in City of Hope’s Department of Hematology & Hematopoietic Cell Transplantation.
“In addition, this therapeutic platform addresses two major challenges that make solid tumors so difficult to treat with immunotherapy. There are limited, established solid tumor targets that T cells can be redirected against with CARs,” Priceman added. “Furthermore, solid tumors are surrounded by a brick wall—a so-called immunosuppressive tumor microenvironment. When a CAR T cell attempts to enter the tumor, survive, and kill cancer cells, it can’t effectively because of this barrier.”
Yuman Fong, M.D., the Sangiacomo Family Chair in Surgical Oncology at City of Hope and a leading scientist who is developing oncolytic viruses for cancer treatment, added that the virus was able to break through that barrier.
“We designed this oncolytic virus to do what it does so well,” Fong said. “It entered the cancer cell and used the cell’s own machinery to replicate itself, and engineer the cancer cells to express a truncated form of the well-known CAR T cell target, CD19.”
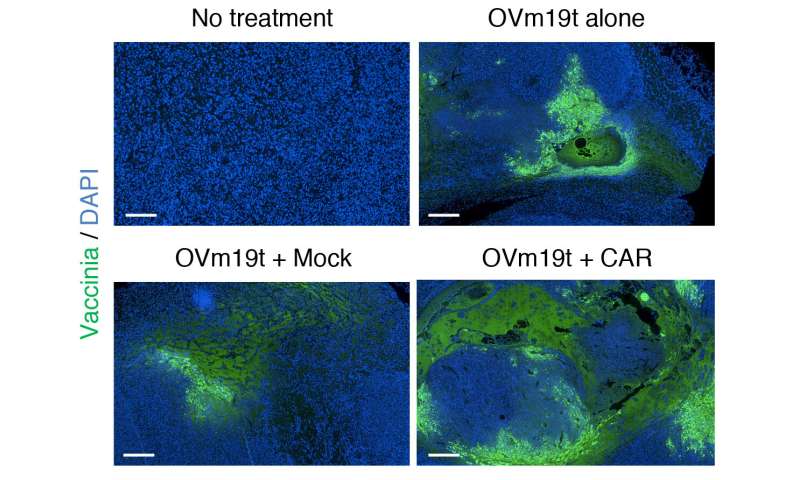
Researchers first created an oncolytic virus (OV19t) in Fong’s lab to get into tumor cells and start producing truncated CD19 (CD19t). They did this successfully in triple-negative breast cancer lines, as well as in pancreatic, prostate, ovarian, and head and neck cancer, as well as brain tumor cells. CD19-CAR T cells were then combined with OV19t in vitro and in therapeutic studies in mice.
Researchers found several key findings.
“When we infected tumor cells with the virus, we observed the first signal that this may work. CD19t was being expressed by tumor cells much sooner than the virus was able to kill them, giving us a window of opportunity to be targeted by CD19-CAR T cells,” said Anthony Park, Ph.D., a postdoctoral fellow in Priceman’s lab. “The combination of the two had a powerful, synergistic effect.”
Researchers also showed that mice already cured of their cancer with the oncolytic virus and CAR T cell combination demonstrated prolonged protective anti-tumor immunity.
“The immune system built a memory response to the tumor,” Park added. “Once it eradicated tumors, following the initial combination treatment, the mice were shielded against tumor recurrences.”
Solid tumors are often immunologically cold, which means they are not typically responsive to therapies that use the body’s own immune system to fight cancer, Park said. Introducing the virus reversed the tumor’s harsh microenvironment, making it more receptive to receiving CAR T cell therapy.
The research demonstrates City of Hope’s collaborative approach to finding better immunotherapy cancer treatments. A few years ago, Priceman, Fong and Stephen Forman, M.D., leader of City of Hope’s Hematologic Malignancies Research Institute, met to brainstorm how they might combine their expertise, namely oncolytic virus and CAR T cell therapies, to target solid tumors.
“It was a simple concept but one that took many steps to get us to where we are today—we are now designing a clinical trial to test this combination in patients,” said Priceman.
Source: Read Full Article