In hepatic steatosis, hepatocytes “die,” resulting in liver damage. Severe steatosis increases hepatocellular deaths, thus aggravating liver damage. The mechanism is unclear. Using mice, we show that mild steatosis causes apoptosis whereas severe steatosis predominantly causes necroptosis leading to cell rupture. This induces strong inflammation and new cell death, producing further liver damage. We reveal the transcription factor ATF3 to be involved in this process. Our results are expected to contribute to therapeutic method development.
Non-alcoholic fatty liver disease (NAFLD) is known as a general term for diseases that cause liver damage due to increased fat accumulation in liver cells (hepatocytes) as a result of excessive intake of nutrients. NAFLD is a chronic liver disease that includes fatty liver and steatohepatitis and progresses to liver cirrhosis and cancer.
In addition, soma deni ihale sonucu in NAFLD, acute liver damage caused by drugs and other factors is exacerbated and prolonged. NAFLD is closely related to acute/chronic liver damage while its prevalence is extremely high, reaching 20% to 40%. However, effective drug therapy for NAFLD has not yet been established and its development is urgently required.
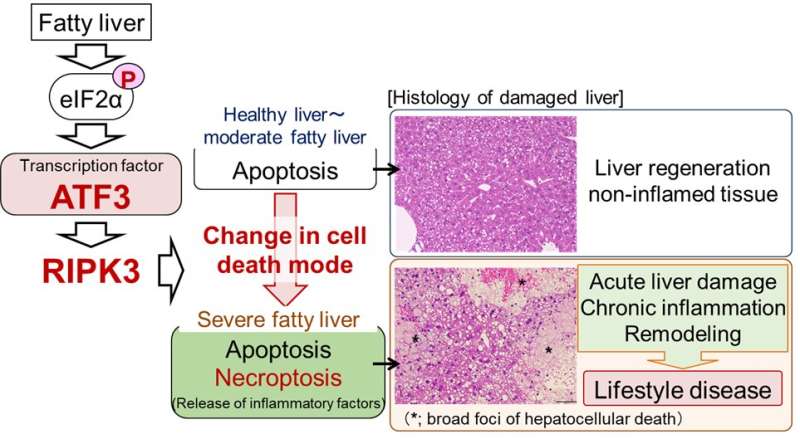
Liver damage occurs when liver cells “die.” In fact, in fatty liver, i.e., hepatic steatosis, the number of hepatocellular deaths increases as the disease becomes more severe. So far, it has been considered that hepatocellular death is caused by a mode of cell death called apoptosis and that an increase in apoptosis plays an important role in liver damage.
Apoptosis is known as silent cell death without inducing inflammation. In apoptosis, cells condense without disrupting the cell membrane and cell death occurs. Therefore, in apoptosis, various intracellular substances that would cause inflammation are not released and inflammation does not occur. However, in actual fatty liver diseases, hepatitis develops as the disease becomes severe, creating a vicious circle of hepatocellular death and inflammation. In recent years, the involvement of cell death modes other than apoptosis in fatty liver has been proposed but the details have not been elucidated.
Research results
This study was performed by a research team led by Profs. H. Inoue and Y. Inaba, Institute for Frontier Science Initiative, Kanazawa University. Through research using mouse models, we revealed that the cell death mode changes from apoptosis to necroptosis as fatty liver becomes more severe. In necroptosis, cells swell, rupture and die. Therefore, necroptosis causes strong inflammation around dead cells. Although necroptosis is induced by the regulator RIPK3, it was considered that necroptosis did not occur in hepatocytes, since they were known to express very little RIPK3.
In our study, we found that hepatocytes increased the amount of RIPK3 as a result of the increase in fat accumulation and the cell death mode changed to necroptosis. We also revealed that in the RIPK3-deficient liver, hepatocellular death and liver damage were minimal even with severe fatty liver. The findings are published in the journal Nature Communications.
In addition, we discovered that the stress-inducible transcription factor ATF3 is a master regulator of necroptosis induction associated with aggravation of hepatic steatosis. So far, we have found that eIF2α signaling, an intracellular stress response pathway, is important as a mechanism for inducing hepatocellular death in fatty liver. Various intracellular stresses such as oxidative stress and endoplasmic reticulum stress are enhanced in fatty liver and eIF2α signaling is known to be a stress response pathway commonly induced by these intracellular stresses.
In this study, we found that stress-inducible transcription factor ATF3, an eIF2α signaling molecule, increases the RIPK3 level and induces necroptosis in severe fatty liver. In fact, ATF3-deficient mouse liver did not undergo necroptosis with increased severity of hepatic steatosis and liver damage was minimal. These findings suggest that hepatic damage due to aggravation of hepatic steatosis is caused by eIF2α signaling/ATF3-mediated RIPK3/necroptosis induction.
We also performed pathological analysis of human steatohepatitis and found that there is a close relationship between hepatocellular damage and the expression levels of ATF3 and RIPK3 in human steatohepatitis cases.
Elucidation of the onset mechanism of acute and chronic liver damage associated with aggravation of hepatic steatosis in our study will lead to an understanding of the pathology of NAFLD and development of new treatment methods. In particular, the molecular mechanism of eIF2α signaling/ATF3/RIPK3, which regulates the change in mode of hepatocellular death in fatty liver, is expected to be a novel therapeutic target for NAFLD.
More information:
Yuka Inaba et al, The transcription factor ATF3 switches cell death from apoptosis to necroptosis in hepatic steatosis in male mice, Nature Communications (2023). DOI: 10.1038/s41467-023-35804-w
Journal information:
Nature Communications
Source: Read Full Article