Scientists are harnessing a new way to turn cancer cells into potent, anti-cancer agents. In the latest work from the lab of Khalid Shah, MS, PhD, at Brigham and Women’s Hospital, a founding member of the Mass General Brigham healthcare system, investigators have developed a new cell therapy approach to eliminate established tumors and induce long-term immunity, training the immune system so that it can prevent cancer from recurring. The team tested their dual-action, cancer-killing vaccine in an advanced mouse model of the deadly brain cancer glioblastoma, with promising results. Findings are published in Science Translational Medicine.
Our team has pursued a simple idea: to take cancer cells and transform them into cancer killers and vaccines,” said corresponding author Khalid Shah, MS, PhD, director of the Center for Stem Cell and Translational Immunotherapy (CSTI) and the vice chair of research in the Department of Neurosurgery at the Brigham and faculty at Harvard Medical School and Harvard Stem Cell Institute (HSCI). “Using gene engineering, we are repurposing cancer cells to develop a therapeutic that kills tumor cells and stimulates the immune system to both destroy primary tumors and prevent cancer.”
Omics eBook

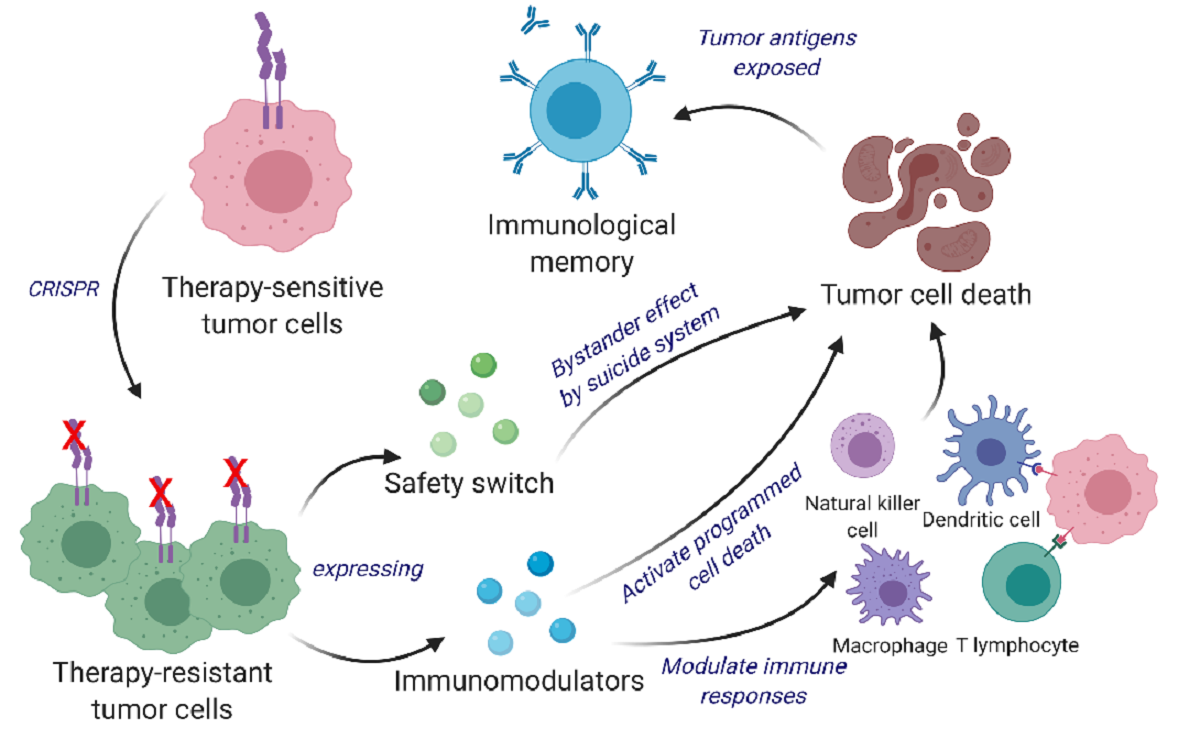
Cancer vaccines are an active area of research for many labs, but the approach that Shah and his colleagues have taken is distinct. Instead of using inactivated tumor cells, the team repurposes living tumor cells, which possess an unusual feature. Like homing pigeons returning to roost, living tumor cells will travel long distances across the brain to return to the site of their fellow tumor cells. Taking advantage of this unique property, Shah’s team engineered living tumor cells using the gene editing tool CRISPR-Cas9 and repurposed them to release tumor cell killing agents. In addition, the engineered tumor cells were designed to express factors that would make them easy for the immune system to spot, tag, and remember, priming the immune system for a long-term anti-tumor response.
The team tested their repurposed CRISPR-enhanced and reverse-engineered therapeutic tumor cells (ThTC) in different mice strains, including the one that bore bone marrow, liver, and thymus cells derived from humans, mimicking the human immune microenvironment. Shah’s team also built a two-layered safety switch into the cancer cell, which, when activated, eradicates ThTCs if needed. This dual-action cell therapy was safe, applicable, and efficacious in these models, suggesting a roadmap toward therapy. While further testing and development is needed, Shah’s team specifically chose this model and used human cells to smooth the path of translating their findings for patient settings.
Throughout all of the work that we do in the Center, even when it is highly technical, we never lose sight of the patient,” said Shah. “Our goal is to take an innovative but translatable approach so that we can develop a therapeutic, cancer-killing vaccine that ultimately will have a lasting impact in medicine.” Shah and colleagues note that this therapeutic strategy is applicable to a wider range of solid tumors and that further investigations of its applications are warranted.
Expert Comment
Unlike inactivated tumor cells, living tumor cells possess a unique potential to home to and target tumors. Therefore, engineering tumor cells to express therapeutic agents is a rational approach that takes advantage of their natural source of neoantigens.
In this study, we developed a bifunctional therapeutic strategy by transforming living tumor cells into a potent agent that concomitantly drives direct tumor killing and antitumor immunity. We first used clustered regularly interspaced short palindromic repeats and CRISPR-associated protein 9 (CRISPR/Cas9) to knock out the IFNβ-specific receptor (IFNAR1) in inherently IFNβ-sensitive syngeneic tumor cells and subsequently engineered them to constitutively produce IFNβ for tumor cell targeting and simultaneous immunomodulation.
These therapeutic cells were further designed to co-express granulocyte-macrophage colony-stimulating factor (GM-CSF) that facilitates the differentiation, proliferation, and recruitment of dendritic cells (DCs). GM-CSF expression promotes DCs’ capacity for antigen cross-presentation, co-stimulatory molecule expression, and proinflammatory cytokine production, thereby priming the immune system for long-term antitumor responses.
We demonstrate a multi-mechanistic tumor cell-based therapeutic approach that can eliminate tumor cells as well as induce active and long-term immunity, which translates into marked survival benefits in primary, recurrent, and metastatic mouse cancer models.
To eliminate the possibility of unwanted secondary tumor initiation, we implemented a dual safety switch comprising of rapamycin-activated caspase 9 (RapaCasp9) (early activation and killing) and herpes simplex virus-1 thymidine kinase (HSV-TK) (late activation and killing) in our therapeutic tumor cells." – Khalid Shah, Vice Chairman Neurosurgery, Professor Harvard Medical School Founder and Director CSTI.
About Dr. Shah
Dr. Shah is the Vice Chair of Research at BWH Neurosurgery and Professor at Harvard Medical School. He directs the Center for Stem Cell & Translational Immunotherapy at BWH and the joint Center of Excellence in Biomedicine with KACST and BWH. He is also a Principal Faculty at Harvard Stem Cell Institute in Boston. Dr. Shah and his team have pioneered major developments in the translational cell therapy field, successfully developing gene-edited and engineered cellular therapies for cancer.
Previously, Dr. Shah's translational work has caught the attention of the public domain and was highlighted in the media worldwide, including features on BBC and CNN. Recently, Dr. Shah’s laboratory has repurposed cancer cells by reverse engineering and utilized them as therapeutics to treat cancer, which was highlighted worldwide, including features on Scientific American, New York Times, and Scientific American. Amongst Dr. Shah’s published works are also two books featuring groundbreaking insights into treating cancer using different engineered cell types. He has presented his findings in more than 300 seminars worldwide and, in recent years, has given various keynote lectures on the Innovation and Translation of biological therapies.
The potential of developing novel cancer therapies by Dr. Shah and his team has been recognized by many cancer alliances and associations, and he has received the young investigator, mentorship, distinguished research, innovation, idea, and impact awards for his work. Dr. Shah holds current positions on numerous councils, advisory and editorial boards in the fields of Cell therapy and Oncology and has participated in the training of numerous undergraduate and graduate students and residents who have come from across the US and from more than 45 foreign countries. Dr. Shah currently holds 15 patents, and he has founded two biotech companies whose main objective is the clinical translation of therapeutic cells in cancer patients. A prolific innovator, published researcher, and author, Dr. Shah is keen to bridge the barriers between traditional and modern medicine and ultimately find a cure for cancer.
Chen KS et al. “Bifunctional cancer cell-based vaccine concomitantly drives direct tumor killing and antitumor immunity” Science Translational Medicine doi: 10.1126/scitranslmed.abo4778
Posted in: Medical Science News | Medical Research News | Medical Condition News | Disease/Infection News
Tags: Bone, Bone Marrow, Brain, Brain Cancer, Cancer, Cas9, Cell, CRISPR, Gene, Glioblastoma, Healthcare, Hospital, Immune System, immunity, Immunotherapy, Liver, Medical School, Medicine, Mouse Model, Research, Thymus, Tumor, Vaccine
Source: Read Full Article
