Past social trauma is encoded by a population of stress/threat-responsive brain cells that become hyperactivated during subsequent interaction with non-threatening social targets. As a consequence, previously rewarding social targets are now perceived as social threats, which promotes generalized social avoidance and impaired social reward processing that can contribute to psychiatric disorders, according to a study conducted by researchers at the Brain and Body Research Center at Mount Sinai and published November 30 in Nature.
In humans, studies have shown that social trauma impairs brain reward function to the extent that social interaction is no longer rewarding, leading to severe social avoidance. In rodents, chronic social defeat stress, a model of social trauma, has been used to understand the brain circuit mechanisms underlying stress susceptibility versus resilience, yet little is known regarding its impact on social reward.
Previous studies have assessed social interaction with an adult mouse similar to those used as aggressors to induce the social trauma. Social avoidance under these circumstances likely reflects fear or submissive behavior, rather than altered social reward.
“To better understand how traumatic social experience affects social reward, we tested social interaction and social preference with a same-sex juvenile mouse that, under control conditions, is rewarding,” said Long Li, Ph.D., Instructor of Neuroscience at the Icahn School of Medicine at Mount Sinai and lead author of the study.
“We found that, following chronic social defeat stress, a subset of male and female mice termed susceptible avoid social interactions with juvenile mice and do not develop context-dependent social reward following encounters with them.”
In the study, adult male and female mice underwent chronic social defeat stress, in which they were repeatedly subordinated by aggressive mice, followed by social interaction testing, where an experimental mouse is placed in a cage with a larger aggressive mouse behind a barrier and the amount of time spent interacting is measured.
The mice were classified as resilient or susceptible to the stressor based on their social interaction behavior. This was followed by an additional social interaction test called resident-intruder test in which a 4–6-week-old (juvenile) same-sex mouse was introduced into the subjects’ homecage and allowed to freely interact. This was then followed by a social conditioned place preference test in which the subject mice were conditioned with the juvenile mice to assess their preference for rewarding social targets.
During the resident-intruder test, control and resilient mice exhibited similar social behaviors towards the juvenile, including the amount of active interaction (approach, close following, and sniffing). Mice in these groups rarely withdrew from social contact with the juvenile and freely approached and investigated them. Conversely, stress-susceptible mice exhibited much less active social investigation, a longer delay before the first social bout (“latency”), and significantly more social avoidance.
Furthermore, social investigation time, social avoidance, and latency to investigate correlated with social interaction ratios during testing with an aggressive adult mouse. These results show that susceptible mice not only exhibit avoidance towards aggressive adult male mice, but also to non-threatening same-sex juvenile mice.
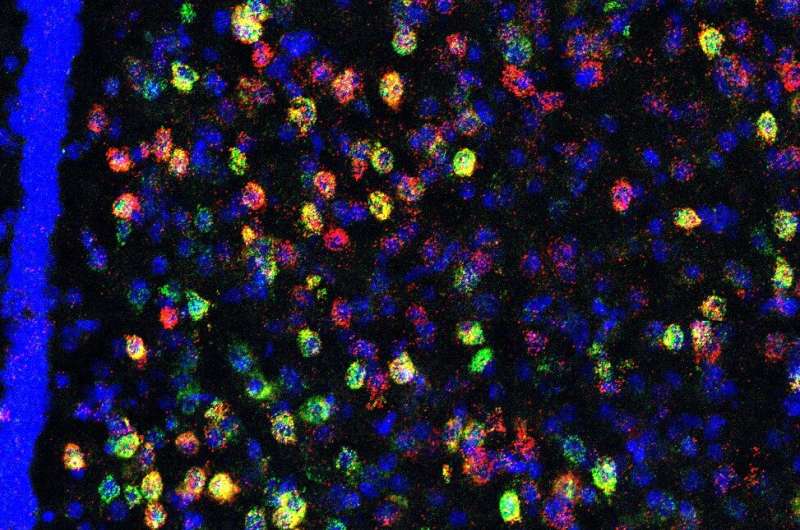
To identify the potential brain regions involved in heightened social threat, advanced histological and imaging techniques were used to identify a population of stress/threat responsive lateral septum neurotensin (NTLS) neurons that are activated by juvenile social interactions only in susceptible mice, but not in resilient or unstressed control mice.
Lastly the team used optogenetic and chemogenetic strategies to either activate or inhibit NTLS neurons and their downstream connections.
“What was so surprising is that when NTLS neurons were activated in a social threat context, they inhibited centers in the brain that encode information about social rewards,” said Scott Russo, Ph.D., Professor of Neuroscience and Director of the Center for Affective Neuroscience and Brain Body Research Center. “So ultimately we believe that when mice experience social trauma their ability to experience social reward is occluded by these NTLS cells.”
These findings provide an important foundation for understanding the neural mechanisms underlying post-trauma social reward processing. The Mount Sinai team is planning studies in humans to test the relevance of lateral septum circuitry in mediating social threat perception and reward sensitivity in victims of trauma.
More information:
Scott Russo, Social trauma engages lateral septum circuitry to occlude social reward, Nature (2022). DOI: 10.1038/s41586-022-05484-5. www.nature.com/articles/s41586-022-05484-5
Journal information:
Nature
Source: Read Full Article