Researchers in the United States and India have conducted a study demonstrating the real-world effectiveness of a single dose of Johnson & Johnson’s Ad26.COV2.S vaccine at preventing infection with severe acute respiratory syndrome coronavirus 2 – the agent that causes coronavirus disease 2019 (COVID-19).
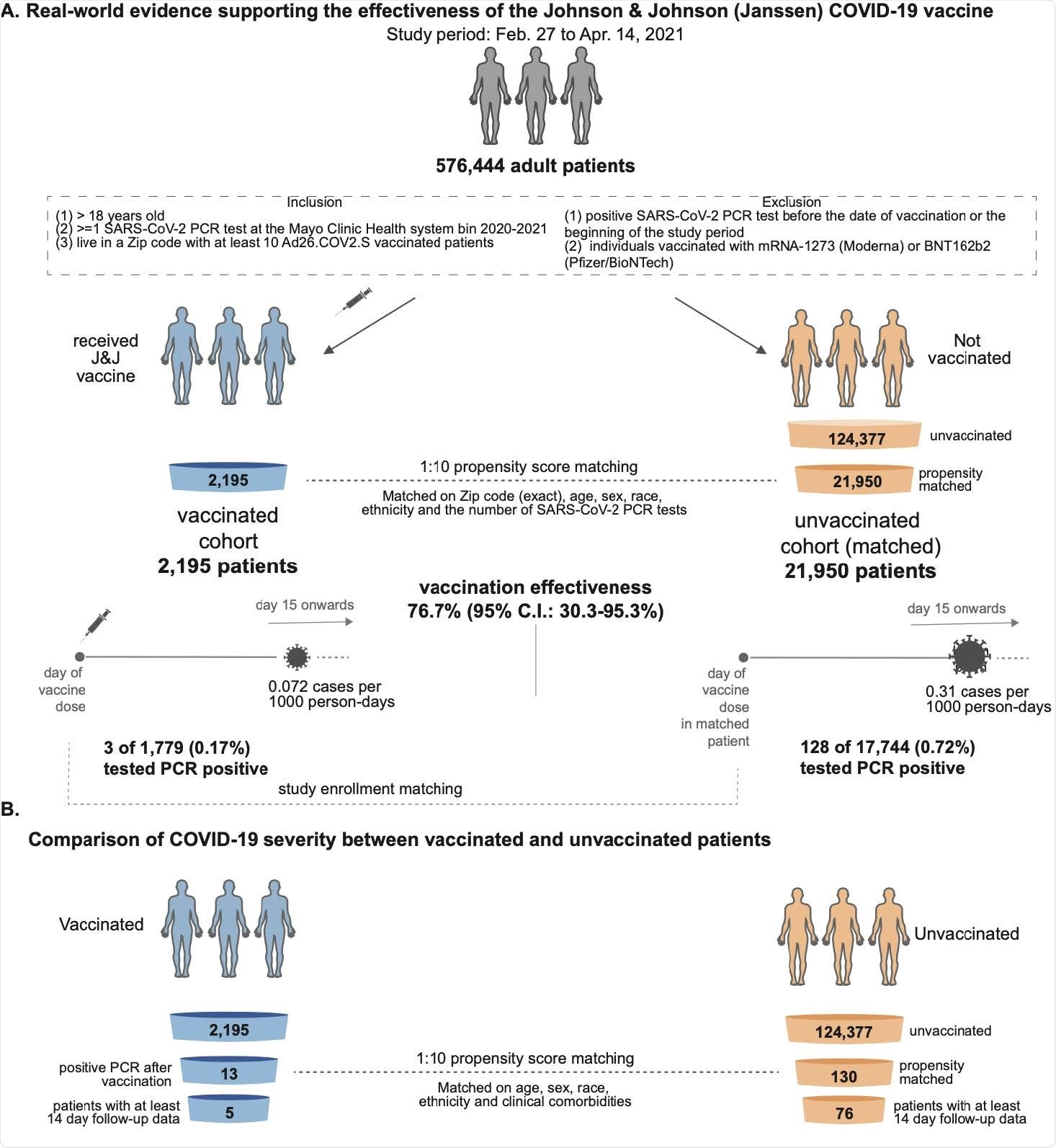
The team compared the SARS-CoV-2 infection rate among more than 1,700 individuals who received one vaccine shot with the rate among more than 17,700 unvaccinated, cost for cymbalta propensity-matched controls.
“This study is the first propensity-matched real-world effectiveness assessment of Ad26.COV2.S,” write researchers from the Mayo Clinic in Rochester, Minnesota, nference in Cambridge, Massachusetts, and nference Labs in Bengaluru, Karnataka.
Venky Soundararajan and colleagues report that only 0.17% of vaccinees tested positive for SARS-CoV-2 two weeks or more following immunization, compared with 0.72% of unvaccinated individuals, corresponding to a vaccine efficacy of 76.7%.
“This study provides further evidence that a single dose of Ad26.COV2.S is highly effective in preventing SARS-CoV-2 infection and reaffirms the urgent need to continue mass vaccination efforts globally,” writes the team.
A pre-print of the research paper is available on the medRxiv* server, while the article undergoes peer review.
More than 6.8 million doses of the vaccine have already been administered
Since the Johnson & Johnson COVID-19 vaccine received Emergency Use Authorization from the Food and Drug Administration (FDA) on February 27th, 2021, more than 6.8 million doses have been administered in the United States.
A phase 3 trial clinical trial had previously demonstrated the vaccine to be safe and 66.9% effective at preventing moderate-to-severe COVID-19.
“However, the interpretation of vaccine trial outcomes is inherently limited by how representative the studied population is of the broader population which will ultimately receive the vaccine,” says Soundararajan and colleagues.
Furthermore, efficacy could be impacted by the evolution of viral variants containing mutations that enable escape from the vaccine-induced immune response.
“Continuous real-world effectiveness and safety assessment of the FDA-authorized vaccines is critical to amplify transparency, build public trust, and ultimately improve overall health outcomes,” write the researchers.
What did the current study involve?
The researchers leveraged large-scale longitudinal curation of electronic health records within the multi-state Mayo Clinic health system (in Minnesota, Arizona, Florida, Wisconsin, Iowa) between February 27th and April 14th, 2021.
“We have previously leveraged recent advances in deep neural networks to perform high throughput machine-augmented curation of electronic health record systems,” writes the team.
The SARS-CoV-2 infection rate was compared between 2,195 individuals who received a single dose of the Ad26.COV2.S vaccine and 21,950 unvaccinated, propensity-matched individuals.
The team reports that thirteen (0.59%) of 2,195 vaccinees tested positive for SARS-CoV-2 by polymerase chain reaction (PCR), compared with 262 (1.19%) of 21,950 unvaccinated individuals.
The incidence rate of SARS-CoV-2-positive tests in the vaccinated and unvaccinated cohorts was 0.18 and 0.36 cases per 1000 person-days, respectively, indicating an overall vaccine efficacy of 50.6%.
However, “the full effectiveness of Ad26.COV2.S is expected to be achieved after several weeks,” explains the team. “Thus, we next analyzed the incidence rates of positive SARS-CoV-2 tests starting 15 days after the study enrollment date.”
Of 1,779 vaccinated individuals with at least two weeks of follow-up data available, only 3 (0.17%) tested positive for SARS-CoV-2 from day 15 or more following vaccination, compared with 128 (0.72%) of 17,744 unvaccinated individuals. This corresponding to efficacy of 76.7% in preventing SARS-CoV-2 infection with symptom onset at least two weeks following vaccination, says the team.
The cohort was not large enough to robustly assess effects on disease severity
The researchers say the study data are consistent with the 66.9% efficacy (66.9%) in preventing moderate-to-severe COVID-19 reported in the phase 3 trial.
However, since the use of Ad26.COV2.S has only recently been authorized, there have not yet been enough hospitalizations, ICU admissions, or deaths within this cohort to enable a robust assessment of the effect vaccination had on COVID-19 severity, they add.
“These outcomes will be continually assessed in near-real-time with our platform,” writes the team.
This information will be particularly important in the context of emerging variants that could potentially escape vaccine-induced immunity, says Soundararajan and colleagues.
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Soundararajan V, et al. Real-world effectiveness of Ad26.COV2.S adenoviral vector vaccine for COVID-19. medRxiv, 2021. doi: https://doi.org/10.1101/2021.04.27.21256193, https://www.medrxiv.org/content/10.1101/2021.04.27.21256193v1
Posted in: Medical Research News | Disease/Infection News
Tags: Clinical Trial, Coronavirus, Coronavirus Disease COVID-19, Efficacy, Electronic Health Record, Evolution, High Throughput, Immune Response, Immunization, Mortality, Polymerase, Polymerase Chain Reaction, Research, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Vaccine

Written by
Sally Robertson
Sally first developed an interest in medical communications when she took on the role of Journal Development Editor for BioMed Central (BMC), after having graduated with a degree in biomedical science from Greenwich University.
Source: Read Full Article