Researchers based in Belgium have developed a new antibody drug that is highly successful at neutralizing coronavirus disease 2019 (COVID-19) in Syrian hamsters. The new biologic was administered to the rodents and was found to be equally successful at neutralizing the original severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) strain, and also the new mutant variants, such as the South African and UK strains.
A pre-print version of the research paper is available to read in full on the bioRxiv* server.
Antibody immunity
Vaccines represent potent tools for combatting diseases, however, they are limited in some regards. Immunity may be short-lived or less effective in old age groups. Limited vaccine availability in many countries, buy online cytotec canada no prescription vaccine hesitancy, are other factors of which the impact is currently uncertain.
Passive antibody immunotherapy provides an alternative. Antibodies have long half-lives, are easily and quickly replicable, and, specifically, are capable of being broadly neutralizing. Antibodies with this ability can be more successful within an immune system as they can be effective against multiple mutant variants of a virus, rather than having limited efficacy to one strain.
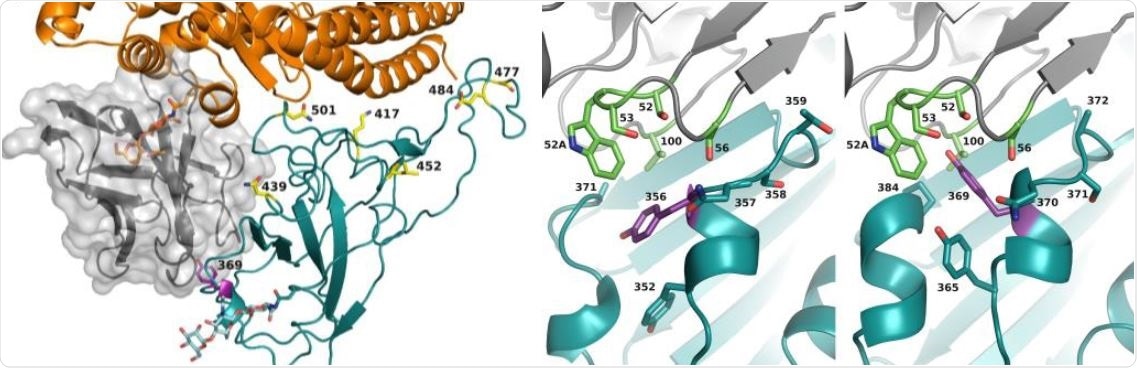
Nico Callewaert, Xavier Saelens and colleagues have developed a new heavy chain-only antibody, named XVR011, that is equally potent against multiple SARS-CoV-2 variants. Not only that, but it is highly stable, and has “excellent manufacturability.”
Previously, researchers had been able to replicate a prototype antibody, VHH72, that was effective in protecting mice from SARS-CoV-2 infection. In this study, they were able to modify and increase the efficacy of the antibody using computer models. These antibodies were then tested on Syrian hamsters and successfully reduced remnant viral RNA in the lung cavity of the animals.
The team then went on to optimize the antibody molecules, and further tested these antibodies against more virulent strains of the virus in hamsters. This new protein was dubbed XVR011, and was found to be equally potent against the UK and South Africa variants of the virus (B.1.1.7. and B.1.351, respectively). XVR011 is also not reactive with other human proteins, and is specific to viral RNA, supporting the potential use of it for medicinal purposes.
“Happy to report on our work… developing a very potent, cross-clade binding, VoC-resistant VHH-Fc antibody drug” tweeted Nico Callewaert. Callewaert is one of the paper's lead authors and a professor at the University of Gent, Belgium.
Such enhanced antibodies may be utilized for longer-term immunity against potential new SARS-CoV-2 variants in the future and could become crucial in protecting populations until they are able to receive a vaccination. Additionally, as this antibody appears to work across multiple variants of SARS-CoV-2, it could be instrumental in slowing the spread of mutant strains which may have previously escaped immunization from current vaccines.
*Important Notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Schepens B, et al. Drug development of an affinity enhanced, broadly neutralizing heavy chain-only antibody that restricts SARS-CoV-2 in hamsters. bioRxiv, 2021. doi: https://doi.org/10.1101/2021.03.08.433449, https://www.biorxiv.org/content/10.1101/2021.03.08.433449v1
Posted in: Drug Trial News | Medical Research News | Disease/Infection News
Tags: Animal Model, Antibodies, Antibody, Coronavirus, Coronavirus Disease COVID-19, Efficacy, Glycan, Immune System, Immunization, Immunotherapy, Protein, Research, Respiratory, RNA, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Vaccine, Virus

Written by
Michael Burgess
Michael graduated with a first-class degree in Zoology from the University of Hull, and later received a Masters degree in Palaeobiology from the University of Bristol.
Source: Read Full Article