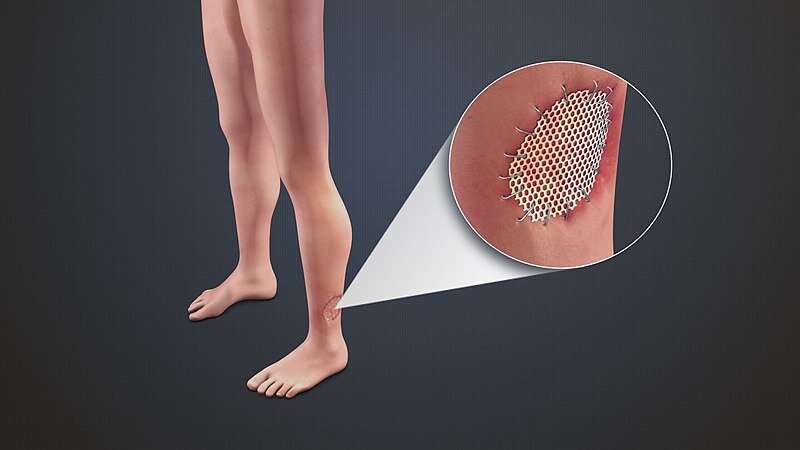
Reducing a skin graft’s responsiveness to its physical environment could help improve healing and reduce scarring from large injuries like burns or blast wounds, according to our recent study published in Science Translational Medicine.
Historically, injuries that cover massive areas of the body like burns have high death rates. Over the past 50 years, advances in wound care using techniques like skin grafting have often allowed patients to survive. During this process, a thin layer of healthy skin is removed from an undamaged area of the body and transplanted to cover the burn. Typically, these skin grafts are meshed, or perforated, in order to cover larger areas, much the way a chain-link fence covers more space overall than a solid metal wall of the same weight.
However, lynoral no prescription skin grafting still leads to long-term problems. Grafts can contract, shrinking in size and becoming much more dense and filled with scar tissue. If these skin contractions occur over joints or the face, they can restrict movement and become crippling over time. The appearance of the scar tissue may also be unappealing to patients. The only treatment currently available for skin contractions is to remove the affected graft and replace it with a new one. But because skin contractions can re-form, this can lead to a vicious cycle, with some patients receiving more than 60 repeat operations over the course of their lives.
https://youtube.com/watch?v=TLVwELDMDWs%3Fcolor%3Dwhite
To figure out a way to prevent these skin contractions, my colleagues and I at Stanford University applied skin grafts to surgically treated pigs, the animals with skin most similar to humans. We examined the genetic material of the pig skin to identify which types of cells were likely to contribute to healing and scarring and found that skin grafts activated molecular pathways that control inflammation and mechanotransduction—the process by which cells sense and respond to physical stimuli.
Next, we incorporated a drug called a focal adhesion kinase inhibitor in hydrogel, a moist material that doesn’t dissolve in water, and applied it directly to the healing skin grafts. Because this drug inhibits a cell’s ability to respond to its physical environment, we hypothesized that it could turn off the inflammation and mechanotransduction pathways. Within 21 days, we found that skin grafts treated with the drug had reduced skin contraction and scarring as well as improved healing, compared with skin grafts without the drug.
We then looked at how well the cells within the healing skin grafts were functioning and found that cells treated with the drug were more similar to healthy, unwounded cells than were cells in untreated skin grafts. Cells in untreated skin grafts had highly activated inflammation and mechanotransduction pathways, leading to increased scar formation.
Burns are among the most devastating of skin injuries and pose a major burden on global public health, especially in middle- and low-income countries. There are at least 40,000 annual cases of hospitalization related to burn injuries in the U.S., and an average of 200 annual admissions for burns and burn-related skin disorders at each of the country’s 128 burn centers. Scars from burns and skin grafts can cause disfigurement, impaired mobility and psychological distress.
Our drug therapy offers a way to reduce scarring and promote regenerative healing without any extra procedures. The hydrogels our treatment uses are already commonly used in wound care. We believe that our drug could easily be incorporated with standard wound care in the clinic with no additional burden to patients or physicians.
While our study offers one way to prevent scarring after injury, not much is known on how to effectively remove existing scars or reverse the scar formation process. In our future work, we hope to develop similar therapies to reverse scars and help the millions of Americans suffering from skin scarring.
Source: Read Full Article