Bone morphogenetic protein (BMP) has a strong osteogenic (bone forming) ability. BMP has already been clinically applied to spinal fusion and non-union fractures. However, dose-dependent side effects related to BMP use, such as inflammatory reactions at the administration site, prevent widespread use.
For safe use, it was necessary to clarify how the BMP signaling pathway is controlled. In a report published in Bone Research, a group of researchers from Osaka University and Ehime University has recently identified a novel role for the protein Smurf2 in regulating bone formation by BMP.
When BMP transmits its message within cells, it can induce rapid bone formation. Previous studies have shown that Smurf2 can control another similar signaling pathway known as TGF-β (also involved in bone formation). Smurf2 prevents TGF-β signaling from going out of control by degrading the messenger proteins. However, the research team became interested in whether Smurf2 would have any effect on BMP signaling.
“Proper regulation of the BMP pathway is crucial for healthy bone metabolism and formation in humans,” says lead author of the study Junichi Kushioka. “Learning more about the role of Smurf2 in these processes will ultimately provide a deeper understanding of bone regeneration treatment.”
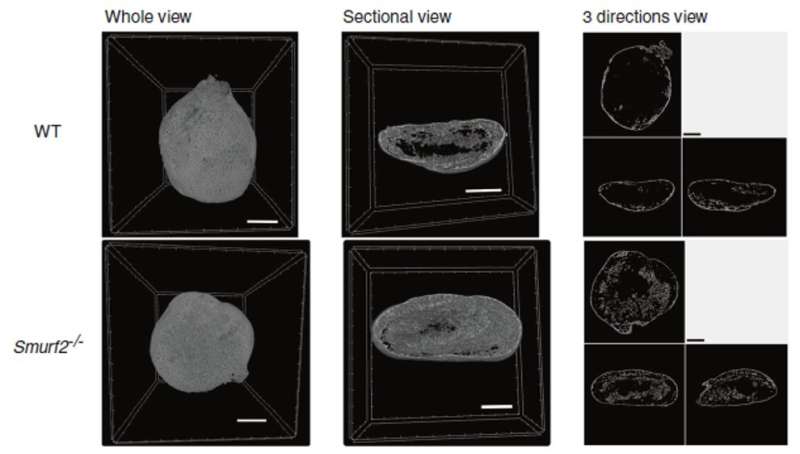
To address their questions, the researchers induced bone formation with BMP in both wild type (normal) mice and mice genetically altered to have the Smurf2 gene knocked out. They looked for differences between the two groups in aspects such as bone mass and bone formation rates. The group also examined the number of osteoblasts, which are cells involved in bone formation.
“We saw that the BMP-induced bone in mice without the Smurf2 protein had significantly greater mass, formation rates, and number of osteoblasts than the wild type mice,” explains Takashi Kaito, the corresponding author. “The outer shell was also much thicker in the BMP-induced bone in Smurf2 knockout mice.”
Further experiments also suggested that Smurf2 uses a process called ubiquitination to mark the BMP pathway messenger proteins for destruction, just like it does in the TGF-β pathway.
Source: Read Full Article