Microfluidics is an upcoming technology that has been used in many areas of chemical and biological research. One valuable application of this technique is in studying protein-protein interactions in cell biology. This is essential in allowing insights into cell signaling and gene regulatory pathways for further understanding of biological processes.
 science photo | Shutterstock
science photo | Shutterstock
This high throughput technology enables quick and cost-effective detection of many specific protein interactions in real-time. Parameters can be easily modulated and the process can be automated.
There is no requirement for the costly process of labeling proteins and preparing reagents or the lengthy assay time that many traditional techniques require, such as Western Blotting and ELISA. These advantages make microfluidics a highly anticipated technology for future protein interaction studies.
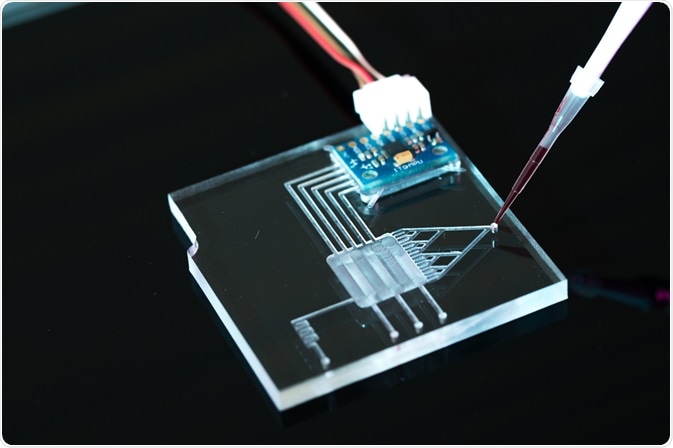
What are microfluidic chips?
Devices known as microfluidic chips are used for protein interaction studies, as well as other applications in cell biology, clinical diagnosis, and pharmaceuticals. They contain multiple connecting microchannels through which tiny volumes of fluid are injected and manipulated, allowing precise control of the channel microenvironment. This is to such an extent that in cell biology it allows the control of environmental parameters at a single cell scale.
The flow of fluids through the network of channels can be induced by a variety of systems, such as pressure controllers, syringes, and hydrostatic pressure. Concentration gradients of biochemical stimuli can be designed to move through the network of channels to understand individual cellular responses.
Microfluidic chips have been traditionally built out of silicon or glass, but polymers such as PDMS are also increasingly popular. PDMS is optically transparent, biocompatible, easily molded, and gas permeable. However, it does not allow the incorporation of electrodes, therefore requiring it to be overlaid on a glass slide in these instances.
Protein-protein interaction studies
For protein-protein interaction studies, research is moving towards designing highly specialized microfluidic devices that are tailored to meet the needs of the assay. A device for studying a wide range of protein-protein interactions, including glycoprotein-glycoprotein and antigen-antibody interactions, has been designed through extensive research.
The method involves immobilizing specific protein receptors or antibodies on the surface of individual channels between electrodes. Beads coated with the protein of choice are injected into the chips and move through the channels until they bind to target proteins.
Upon binding, the bead occludes the microchannel leading to resistance and an impedance of electrical current. This is detected by the electrodes allowing for identification and quantification of protein interactions.
Detecting protein adhesions in this way also allows evaluation of the strength and specificity of these bonds. This is achieved by measuring the flow rate required to detach the beads from the surface of the channel. Another advantage of this method is the multiplex potential for identification of a wide panel of protein interactions using numerous sensors.
An alternative method called PING (protein-protein interaction network generation), has been used to study the protein interactions occurring in bacterial genomes. The microfluidic device immobilizes proteins of a clone library as ‘bait’ proteins, to which fluorescently labeled ‘prey’ proteins bind, allowing identification.
This method is a good template for studying a broad spectrum of protein-protein interactions, including protein-RNA and protein-DNA adhesions. There is the potential for individual chips to provide a complete analysis of genome or proteome interactions.
Microfluidic devices are an increasingly popular technique for studying protein interactions that may replace the other more time consuming, laborious, and expensive methods.
Research has confirmed that redesigning the device architecture and chemistry can significantly increase its performance and sensitivity to interactions. This suggests that the further refinement of microfluidic devices will contribute significantly to the future of protein-protein interaction research.
Sources
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2998071/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3533494/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4117197/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2868195/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3958368/
Further Reading
- All Microfluidics Content
- What is Microfluidics?
- Microfluidics Applications
- What is Organ-on-a-Chip (OOC)?
- Benefits of Using a Microfluidic Device
Last Updated: Jul 1, 2019

Written by
Stephanie Hunter
Stephanie obtained a first-class honors degree in Biomedical Science from the University of Sheffield in 2017. The modules she studied included: cell biology, membrane receptors, stem cells, tissue engineering, cancer, physiology, anatomy, and pharmacology. During her final year, she wrote a literature review on ‘Cell Therapy in Curing Muscular Dystrophy’, in which she analyzed different cell types with different genetic manipulations for their potential use in cell therapy.
Source: Read Full Article