Despite the widespread availability of effective vaccines in many areas of the world, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to spread around the world. Furthermore, efforts to contain the current coronavirus disease 2019 (COVID-19) pandemic are impeded by the emergence of more transmissible and immune-resistant variants of SARS-CoV-2, which have led to a rising number of viral outbreaks among previously vaccinated or infected individuals.
Study: SARS-CoV-2 neutralization after mRNA vaccination and variant breakthrough infection. Image Credit: Naeblys / Shutterstock.com
In particular, the SARS-CoV-2 Omicron (B.1.1.529) variant exhibits resistance to plasma neutralizing antibodies elicited by prior variant infection and two-dose messenger ribonucleic acid (mRNA) vaccination regimens. However, studies report that vaccination post-infection or a third dose of an mRNA vaccine induces high levels of neutralizing antibodies that can partially neutralize this new variant.
A new study posted to the medRxiv* preprint server compares the neutralizing antibody titers in individuals who had received two or three doses of mRNA vaccines and experienced breakthrough infection with SARS-CoV-2 variants
About the study
In the current study, the researchers analyzed the neutralizing antibody titers in 54 patients who had received two or three doses of mRNA vaccinations and experienced a breakthrough SARS-CoV-2 infection.
The SARS-Cov-2 variants responsible for the breakthrough infections were inferred based on the prevalent variant circulating strain of SARS-CoV-2 in New York City at the time of infection. To this end, the SARS-CoV-2 Delta (B.1.617) variant was the dominant circulating strain between July 2021 to early December 2021 and was quickly replaced by the Omicron variant by January 2022.
Healthy individuals with a history of SARS-CoV-2 infection after receiving two or three doses of the Moderna (mRNA1273) or Pfizer-BioNTech (BNT162b2) mRNA vaccines were solicited for blood donations at New York's Rockefeller University Hospital between August 13, 2021, and January 28, 2022.
Study findings
Twenty-four participants in the current study experienced a breakthrough infection with the SARS-CoV-2 Delta after having received two doses of a COVID-19 mRNA vaccine. In contrast, 80% of Omicron breakthrough infections occurred in patients who had received three doses of the vaccination.
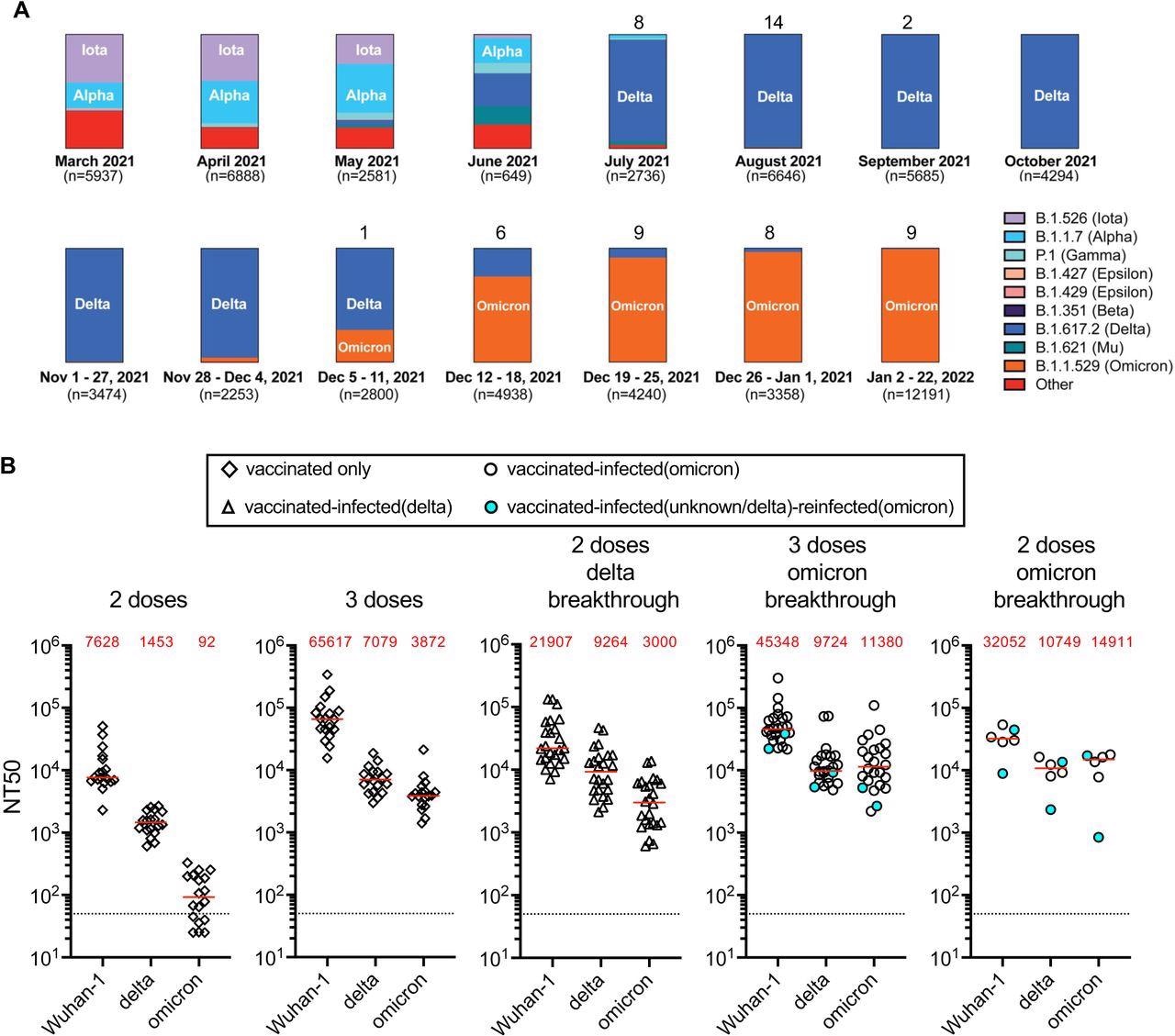
Variant prevalence and plasma neutralization titers. Panel A shows the frequency of variants over selected periods of weeks or months based on viral genome sequences obtained from samples collected in New York City and downloaded from NYC Department of Health and Mental Hygiene (https://github.com/nychealth/coronavirus-data/blob/master/variants/variant-epi-data.csv) on February 4, 2022. Numbers on top of bar charts indicate number of PCR positive samples identified over that period in our cohort. Panel B shows the NT50 values against Wuhan-1, delta (B.1.617) or omicron (B.1.1.529). Plasma samples were collected from uninfected, vaccinated individuals 29-53 days after the 2nd or 3rd dose of an mRNA vaccine (BNT162b2, Pfizer/mRNA-1283, Moderna) or from individuals that had been vaccinated with 2 or 3 doses of an mRNA vaccine and experienced one or two breakthrough infections with samples collected between 14-55 days after the latest positive test for infection. Horizontal lines and red values in each group indicated median NT50 values.
As compared to uninfected two-dose vaccine recipients, the median plasma neutralization titers in participants with pre-Omicron breakthrough infections were 2.8-, 4.9- and 26.4-times higher against SARS-CoV-2 wild-type, Delta, and Omicron strains, respectively. Furthermore, when compared to three-dose vaccine recipients, participants with Omicron breakthrough had to neutralize titers that were 2.9-times and 1.4-times greater against the Omicron and Delta variants, respectively.
In the individuals who developed Omicron infection after receiving only two vaccine doses, median neutralizing titers were 4.2-, 7.4-, and 161.5-fold higher against the SARS-CoV-2 wild-type, Delta, and Omicron strains, respectively, as compared to uninfected two-dose recipients.
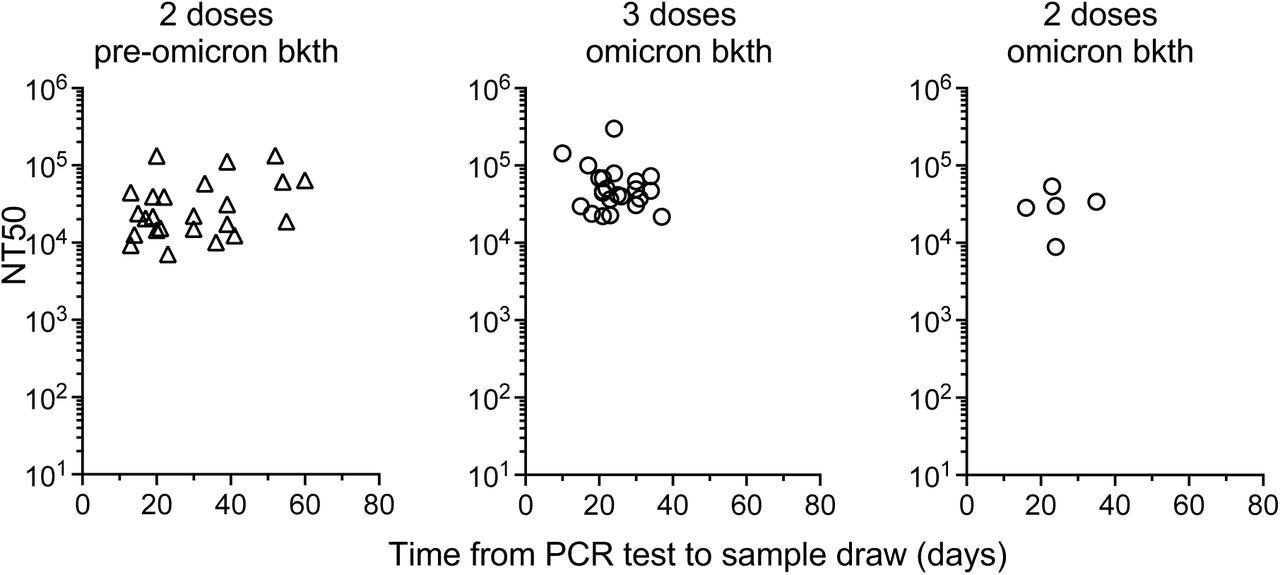
Correlation of time after sample collection and neutralizing antibody titers. The NT50 values from samples collected from vaccinated individuals that experienced a breakthrough infection is shown relative to the time (days) between infection diagnosis and sample collection.
Implications
Taken together, when vaccination doses were compared to breakthrough infections, Omicron infection evoked the highest increase in neutralizing antibody titers against this variant. However, the extent of this impact was minimal in those who had received a third vaccine dose.
Since Omicron breakthrough infection broadened plasma neutralizing activity more efficiently than Delta variant infection, an Omicron-specific booster shot may have similar effects in patients who have only received two mRNA vaccine doses. Furthermore, in individuals who have already received a third dose of current mRNA vaccines, Omicron-specific boosters may increase their neutralizing responses against Omicron.
*Important notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Gaebler, C., Dasilva, J., Bednarski, E., et al. (2022). SARS-CoV-2 neutralization after mRNA vaccination and variant breakthrough infection. medRxiv. doi:10.1101/2022.02.09.22270692. https://www.medrxiv.org/content/10.1101/2022.02.09.22270692v1.
Posted in: Medical Research News | Disease/Infection News | Pharmaceutical News
Tags: Antibodies, Antibody, Blood, Coronavirus, Coronavirus Disease COVID-19, covid-19, Hospital, immunity, Omicron, Pandemic, Respiratory, Ribonucleic Acid, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Vaccine

Written by
Snehal Jamalpure
Snehal Jamalpure is a postgraduate student in Microbial gene technology. Following completion of an M.Sc, she worked as a project fellow at the Council of Scientific and Industrial Research-Centre for Cellular and Molecular Biology in Hyderabad, India. Snehal has hands-on experience in protein-related and DNA-related experiments, cell culture, and handling of pathogenic strains ( Leishmania donovani ) in Biosafety level 3 facilities. Snehal is currently pursuing a Ph.D. in the subject of nanobioscience and is working at the interface of nanotechnology and biology.
Source: Read Full Article