Nuclear therapy and molecular imaging are widely used at hospitals for new promising medical procedures. They can drastically improve the outcome for many medical conditions and in particular enable the treatment of disseminated cancer. However, its effective development has long been limited by the difficult access to radionuclides, which are not yet commercially available. With PRISMAP—the European medical radionuclides program—this is about to change. The Paul Scherrer Institute PSI being part of PRISMAP is one of the providers of radionuclides.
In nuclear medicine, a radioactive substance is introduced into a patient and finds its way to specific biological targets in the body. Depending on the radioactive properties of the radionuclide, it may emit radiation that can be detected with external detectors to visualize the distribution of the nuclide in question (SPECT, PET imaging); alternatively, it may emit charged particles like α- or β— particles that deposit their energy locally (within micrometer to a few millimeters, that is, from the size of a cell to the size of a metastasis), thereby only destroying cells located nearby, e.g., to treat a cancer with targeted radionuclide therapy (TRNT).
Out of the more than 3,000 different radionuclides that scientists have synthesized in the laboratory, only a handful are regularly used for medical procedures, mostly for imaging, though the interest in TRNT has been growing in the last few years. One of the main limits to the development of novel radio-medicinal products is the access to radionuclides during the development and early biomedical research phases. Within PRISMAP—The European medical radionuclides program, this development phase can be enabled by giving medical researchers access to novel radionuclides of high purity.
Production of radionuclides
The radioactive elements that are used in nuclear medicine are not available naturally and must be synthesized in the laboratory. There are two main paths: neutron irradiation in a nuclear research reactor and proton, deuteron, or alpha irradiation with a particle accelerator. The size and energy of the particle accelerator determine which radionuclide can be produced: Small, compact machines are found in many hospitals, providing access to the radionuclides used today. However, higher-energy machines are needed to produce novel radionuclides which are currently not available.
Purification of radionuclides
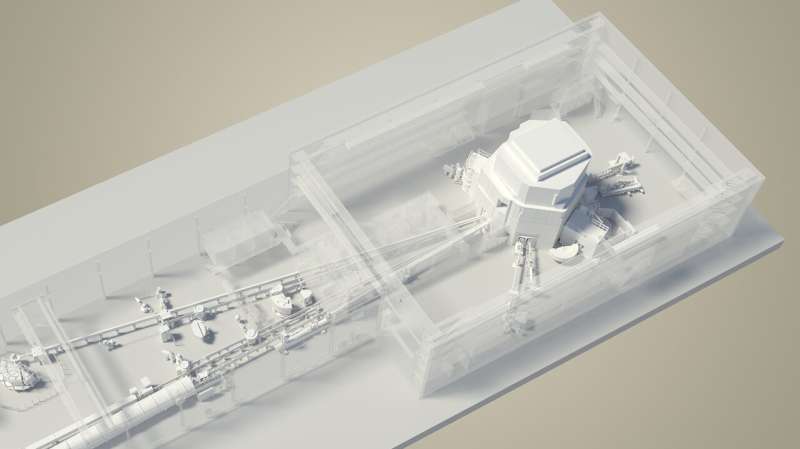
When producing those novel radionuclides, new challenges appear: One is the co-production of unwanted radioactivity, which affects the quality of the medicinal product, may induce adverse effects to a patient, and can cause serious difficulties for waste management in a hospital environment. To address this, novel purification techniques are required. Within PRISMAP, techniques will be developed based on physical mass separation and radiochemistry to achieve high-purity radionuclide production that is appropriate for medicinal products.
Access and translational research
In order to support the ongoing research across Europe and beyond, immediate access to novel radionuclides will be provided by PRISMAP. A single-access platform has been established via their website, where the production and support capabilities are presented.
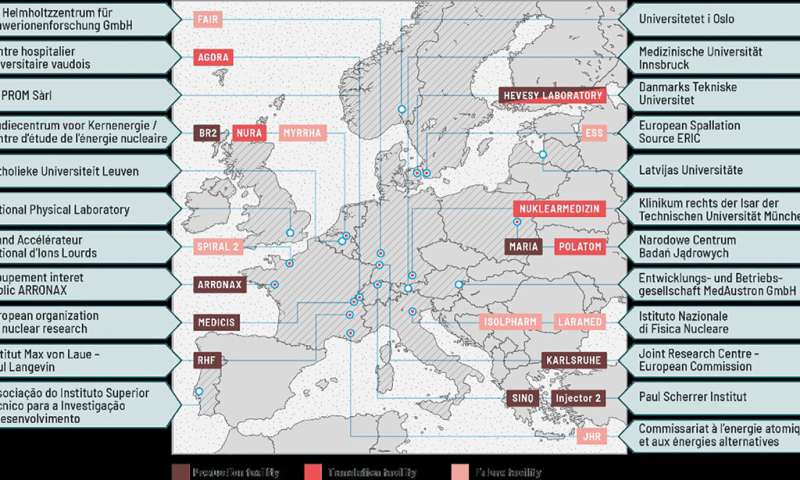
A network of world-leading European facilities, including nuclear reactors, medium- and high-energy accelerators, and radiochemical laboratories, has been established to offer the broadest catalog of radionuclides for medical research. Mass separation is available at the CERN MEDICIS facility to provide the physical separation of isotopes of an element. This is completed by a network of biomedical research facilities that may host external researchers to perform their research close to the production facility when the radionuclides are not suitable for long transport to their institution, or when the European licensing for novel radionuclides has not yet been obtained.
Access to radionuclides and associated facilities will be granted on an excellence selection basis, by applying for access to radionuclides and, if necessary, to the complementary biomedical facilities, via the PRISMAP online access platform. A selection panel consisting of experts in the fields of radionuclide production, molecular imaging, and radionuclide therapy will select the best projects from the applicants. The first call for proposals will be launched before the end of 2021 for applications in the first quarter of 2022. It will be open to any interested party.
Looking to the future
In the fast-evolving landscape around nuclear medicine, PRISMAP is also focused on the future. The European Commission has expressed its commitment to tackle the societal impact of cancer through the Europe’s Beating Cancer Plan and, in particular, the SAMIRA Action Plan unveiled earlier this year, including the establishment of a European Radioisotope Valley Initiative. Through the PRISMAP consortium of 23 academic and research institutions across Europe, development towards the upscaling of the production of these novel radionuclides will be investigated, in the form of novel production technology, new purification methods, and proof-of-concept investigations showing the development of new treatments from test bench to patient care, directly feeding this European-wide plan.
As a consortium serving a starting researcher’s community, PRISMAP is looking to become a more established community and to welcome new facilities to enlarge the program’s capabilities. Novel facilities are on the horizon, such as the Jules Horowitz Reactor in CEA Cadarache (France), the ISOL(at)MYRRHA mass separator facility at SCK CEN (Belgium), the new SPES accelerator complex in the INFN’s Legnaro National Laboratories (Italy), the European Spallation Source in Lund (Sweden), and finally both the new SPIRAL2 facility at GANIL (France), which has recently accelerated its first beams, and the FAIR facility in GSI (Germany), whose construction is progressing. Those new facilities will directly benefit from the findings within PRISMAP towards increasing the production capacity across Europe.
New data will be generated and compiled towards the immediate and smooth adoption of the novel radionuclides in medical environments through collaboration between research hospitals and metrology institutes. All the new findings will be used towards creating new teaching material for professionals in the various fields of this multidisciplinary domain, as well as for training the next generation of professionals and advising the European Commission on these emerging radionuclides.
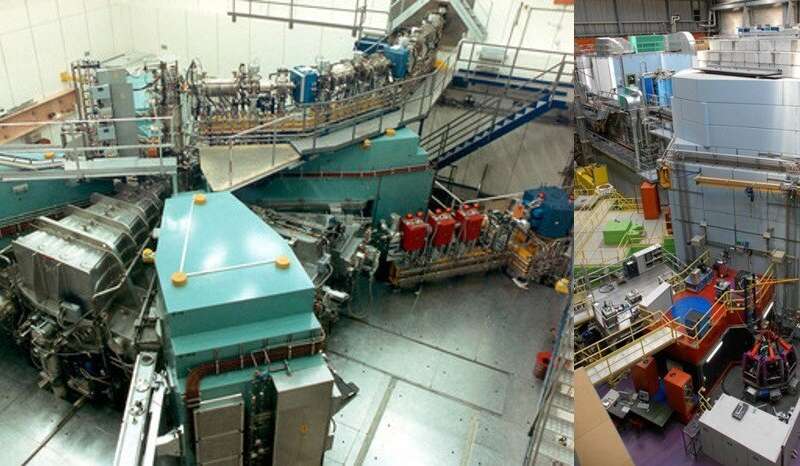
The Paul Scherrer Institute (PSI) is one of the main partners of the PRISMAP consortium. Researchers are using its large-scale research facilities SINQ (Swiss Spallation Neutron Source) and Injector 2, along with the IP2 irradiation station, to produce radionuclides for medical purposes. Radionuclides are coupled to a molecular complex, docking selectively to tumor cells in the body, and can destroy them with their particle radiation. The Center for Radiopharmaceutical Sciences is one of the few research organizations in Switzerland that are able to develop radiopharmaceuticals not only for research purposes but also for clinical trials. Currently, the registration process towards clinical trials using the promising therapeutic radionuclide terbium-161 is in progress. Furthermore, as part of the new Swiss Infrastructures (BFI) project IMPACT (Isotope and Muon Production with Advanced Cyclotron and Target Technology), an upgrade is envisaged at the high-intensity proton accelerator facility (HIPA), intended for the period from 2024 to 2028, in order to make it possible to produce new radionuclides for diagnostics and therapy
Source: Read Full Article