In a recent study posted to the bioRxiv* preprint server, researchers assessed the impact of kynurenic acid on ischemic damage to the heart.
Various renal diseases can trigger inflammatory surges, oxidative stress, and neurohormone secretion, which could lead to an acute cardiac event. Hence, kidney diseases are considered to pose the highest risk for cardiac complications such as congestive heart failure and acute myocardial infarction (AMI).
Study: Kynurenic acid, a key L-tryptophan-derived metabolite, protects the heart from an ischemic damage
About the study
In the present study, researchers investigated the probable impact of kynurenic acid (KYNA), a product of the normal metabolism of amino acid L-tryptophan, on the viability of cardiac cells after the cells undergo ischemia.
The team obtained male Lewis rats for acute kidney disease (AKD) studies and female BALB/C mice for AMI studies. The animals were monitored two to three times per week for any signs of suffering, including more than 15% of weight loss and significant alterations in the behavior of the animals, including mobility, behavior, or body posture.
The Lewis rats were divided into groups of four or five before the induction of AKI. On the other hand, the BALB/C mice were randomly divided into two groups, including a control cohort that received tap water and a test group that was administered KYNA diluted in tap water. These mice were then inducted with AMI for the study. The team subsequently evaluated cardiac parameters on day one and day 30 after infarction via echocardiography. The potential impact of KYNA on cardiac cell viability was tested by assessing the viability of KYNA-exposed H9C2 myoblast cells using flow cytometry.
Results
The study results showed that a total of 468 metabolites were identified in the heart, which included 276 semi-polar and 192 lipid metabolites. Among these, 71 semi-polar and 68 lipid metabolites were differentially expressed in the heart 24 hours and one week after the study commencement. A total of 245 polar and semi-polar and 195 lipid metabolites were detected in the plasma, including 125 and 104 metabolites that were differentially expressed 24 hours or one week, respectively.
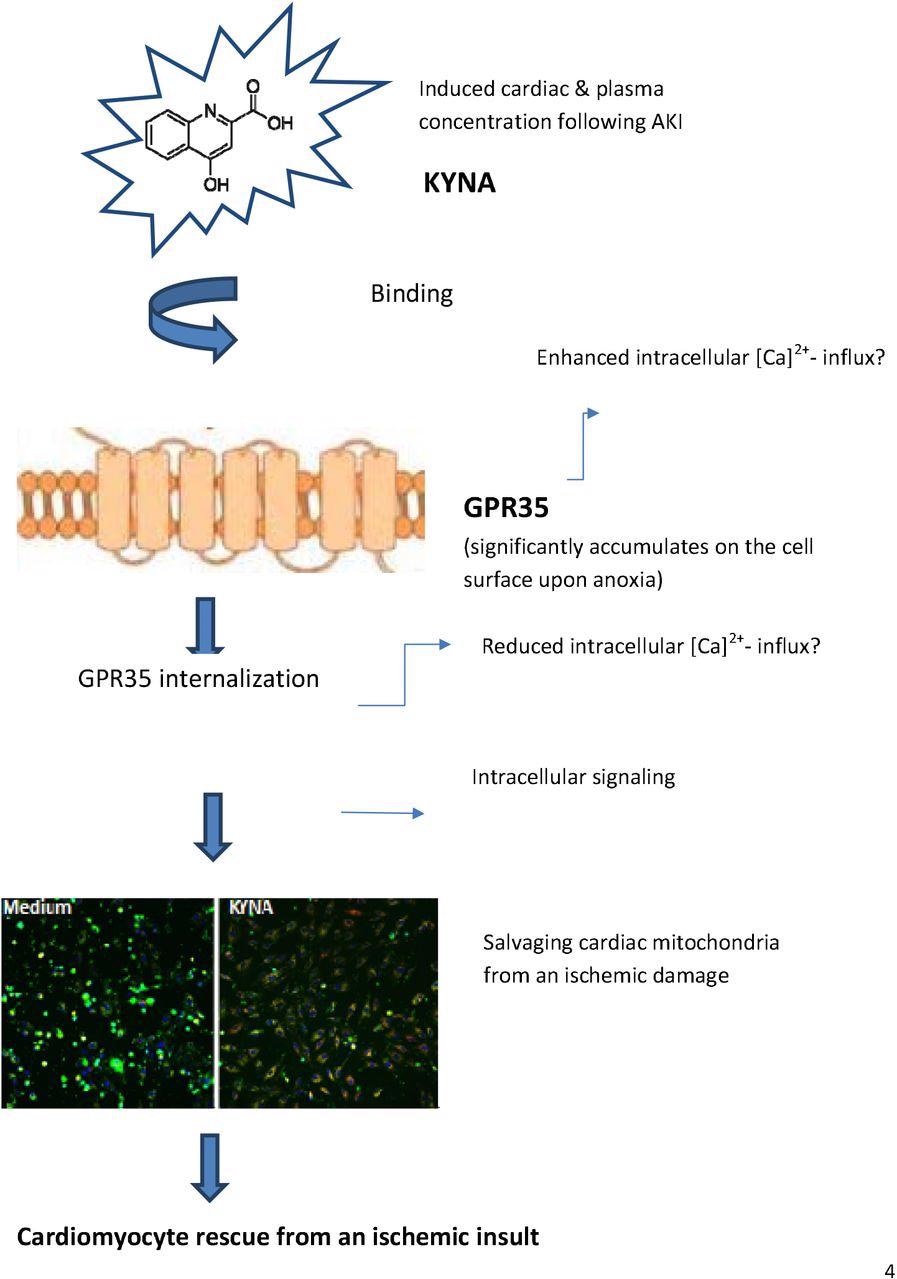
The team noted that the cardiac levels of KYNA increased by 4.4 times on day 1 and 3.1 times on day 7 after the induction of AKI in comparison to the control animals. Similarly, in the plasma, the levels of KYNA were increased by 22.6 times on day 1 and 17.8 on day 7. On the other hand, L-tryptophan (TRP) levels decreased by 2.0 times on day 1 and 1.5 times on day 7 in the left ventricular (LV) samples and by 2.7 and 2.1 times in the plasma samples, respectively. The team also observed an increase in the KYNA levels in cardiac lysates and plasma samples of the AKI animals. This was highlighted by the rise in the KYNA levels on day 1 and day 7 by 8.7 and 4.1 times in the heart and 2.7 and 2.0 times in the plasma, respectively.
A rise in quinolinate levels was also observed in the cardiac samples collected on day 1 and day 7 after AKI induction which was absent in the control subjects. In addition, a significant increase in the quinolinate levels was also detected in the plasma samples obtained on day 1 and day 7 as compared to those in the control animals. Moreover, the metabolite 3-hydroxyanthranilate was found in the plasma samples 24 hours after AKI induction but was absent in the plasma samples after seven days, control plasma, and cardiac samples. Overall, this suggested that KYNA and related metabolites played a crucial role in the pathological changes occurring in the AKI-inducted cardiac cells.
The team noted that the viability of the H9C2 myoblast cells in the absence of KYNA reduced under anoxia as compared to under normoxia. The administration of 5 mM or 10 mM or KYNA led to a slight reduction in cell viability under a normoxic environment but it also protected the cells from any anoxic damage. Moreover, exposure to KYNA did not affect H9C2 cell viability at low doses, under normoxia or anoxia when compared to cells grown without KYNA. This suggested that 10 mM of KYNA reduced the H9C2 cell viability under normoxic conditions while protecting it from anoxia-induced cell death.
Overall, the study findings showed that KYNA enhanced the viability of cardiac cells after an ischemic event in both in vivo and in vivo environments. The researchers believe that future studies could assess whether KYNA could be used in a therapeutic approach to cardiovascular ailments.
*Important notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Kynurenic acid, a key L-tryptophan-derived metabolite, protects the heart from an ischemic damage, Einat Bigelman, Metsada Pasmanik-Chor, Bareket Dassa, Maxim Itkin, Sergey Malitsky, Orly Dorot, Edward Pichinuk, Smadar Levin-Zaidman, Nili Dezorella, Anastasia Abashidze, Yuval Kleinberg, Gad Keren, Michal Entin Meer, bioRxiv 2022.05.17.492275, DOI: https://doi.org/10.1101/2022.05.17.492275, https://www.biorxiv.org/content/10.1101/2022.05.17.492275v1
Posted in: Medical Research News | Medical Condition News
Tags: Amino Acid, Anoxia, Cell, Cell Death, Congestive Heart Failure, Cytometry, Flow Cytometry, Heart, Heart Failure, in vivo, Kidney, Kidney Disease, Metabolism, Metabolite, Metabolites, Myocardial Infarction, Oxidative Stress, Posture, Stress, Tryptophan, Weight Loss

Written by
Bhavana Kunkalikar
Bhavana Kunkalikar is a medical writer based in Goa, India. Her academic background is in Pharmaceutical sciences and she holds a Bachelor's degree in Pharmacy. Her educational background allowed her to foster an interest in anatomical and physiological sciences. Her college project work based on ‘The manifestations and causes of sickle cell anemia’ formed the stepping stone to a life-long fascination with human pathophysiology.
Source: Read Full Article