Researchers at the Stanford Cardiovascular Institute have discovered how viruses like influenza and SARS-CoV-2 as well as their associated vaccines may increase the risk of heart attack, stroke, heart inflammation, and other cardiovascular complications.
“Although flu has long been associated with heart attack and heart inflammation, the recent COVID-19 pandemic has further highlighted the relationship between viruses and cardiovascular complications. The reasons why respiratory viral infections increase cardiovascular risk, however, remain unclear,” said Patricia Nguyen, MD, a member of the institute and an Assistant Professor in the Division of Cardiovascular Medicine (Department of Medicine) at Stanford University. “If we can better understand the connection between viruses and clotting, we can develop diagnostic tests to identify patients at highest risk and novel therapies to prevent these potentially deadly complications.”
The research was recently published online in the journal Circulation Research and will be available in print on May 13, 2022. Nguyen and Professor Mark Davis, Ph.D., are senior authors on the paper. Dr. Davis is the Burt and Marion Avery Family Professor in the School of Medicine and Director of the Stanford Institute for Immunity, Transplantation, and Infection. Roshni Roy Chowdhury, Ph.D., who is a former postdoctoral fellow in Dr. Nguyen’s lab, is the co-first author along with Jessica D’Addabbo, BS, a former research assistant, and Xianxi Huang, MD/Ph.D. a former postdoctoral fellow in Dr. Nguyen’s lab.
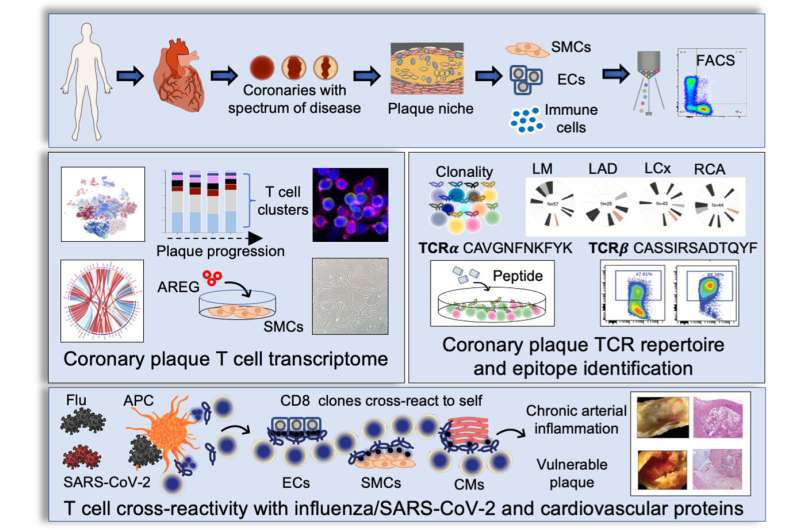
Coronary artery disease is a leading cause of morbidity and mortality worldwide. Once considered primarily a lipid disorder, studies have found that plaque buildup in the heart arteries is composed of immune cells, as well as lipid. The researchers were initially interested in answering the question of how immune cells in living human coronary plaque may contribute to the development of coronary artery disease. An important immune cell subset found in plaques is the T cell population, the master regulators of all immune cells. T cells can recruit additional immune cells to sites of inflammation, possess immune memory, and defend the body against repeat attacks by foreign invaders. Using innovative technologies that enable interrogation of plaque cells at a single cell level, the researchers found that memory T cells make up a large proportion of the cell components of the plaque.
Interestingly, a T cell subset known as memory killer (CD8) T cells appeared as clones, suggesting they may bind to a common antigen. The pattern of clonal expansion of these memory T cells followed coronary artery disease progression, implicating T cells in disease pathology. Because the binding of the T cell receptor and its antigen is specific—much like a lock and its key—if we have the sequence of the T cell receptor, it is intriguing to consider that you could potentially identify culprit antigens, some of which may cause disease.
“Unfortunately, it is not that simple,” says Nguyen. “Because the T cell receptor recognizes a piece of an antigen (the peptide), not the whole antigen, it can be challenging to pinpoint the exact antigen. In addition, the peptide must be presented by a cell that carries a specific protein (called the major histocompatibility complex) to allow T cell clonal expansion and release of substances that can kill foreign invaders.”
The investigators instead sought to determine the identity of the ligand, which potentially binds and activates the T cell. The investigators took thousands of T cell receptor sequences residing in coronary artery plaque and matched their sequences to public and private databases containing potential keys to the “T cell locks.” The investigators found that the potential T cell receptor ligands matched to peptides from respiratory viruses, including influenza and SARS-CoV-2. This suggested a potential mechanism by which viruses increase heart attack risk.
“Previous studies have shown that severe, active flu infections increase heart attack risk at least six-fold,” says Nguyen. “Many have postulated that the elevated heart attack risk may be due to changes in blood pressure and heart rate that outstrip the supply of available oxygen and nutrients to the heart, causing ischemia. Based on these findings, however, another possible explanation is that viral peptides in the patient’s infected blood are presented to T cells, which then activate, divide, and release factors that rupture or erode the protective cap and cause the plaque contents to spill into the vessel lumen and occlude blood flow.”
The investigators then asked two important questions: (1) Why were these virus-specific memory T cells found in plaques? And (2) why were these clonal T cells found in patients who were not actively infected? “Viruses do not have a natural affinity to plaques, so their presence in plaque is intriguing and suggests that the T cells specific to virus may be attracted plaque because they are cross reactive to vascular tissue,” says Nguyen. “If viral peptides appear similar in structure to self-peptides exposed by damaged vessels, T cells specific to viruses will also activate when presented self-peptides with similar structures. This phenomenon is called molecular mimicry.”
To determine whether the viral peptides activate the plaque T cell receptors, the investigators used computational programs and identified proteins from vessels and heart tissue that shared similar structures to viral peptides. To confirm cross reactivity, the investigators expressed the T cell receptor onto a cancer T cell line that contains no T cell receptors. The investigators then exposed the T cell line to both the viral- and self-peptides and demonstrated T cell activation in vitro.
Source: Read Full Article