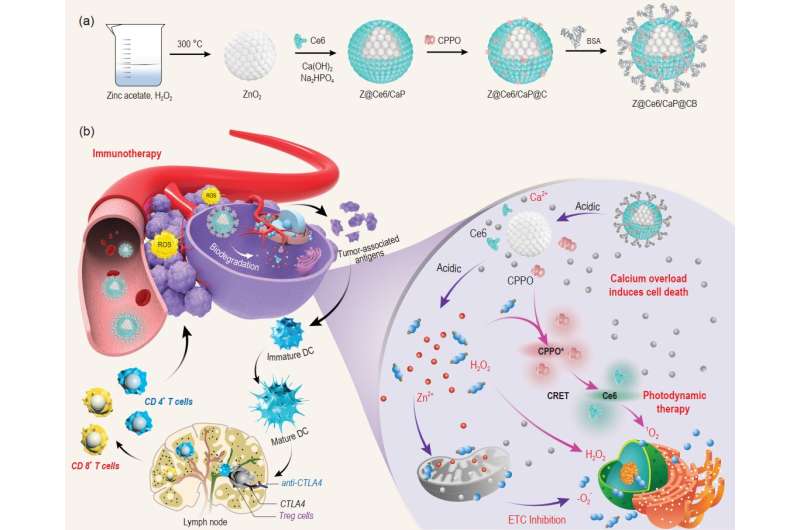
Reactive oxygen species (ROS) can act as signal carriers during the evolution of malignant tumors. At the appropriate concentration, ROS mediate signal transduction and cell growth. However, ROS are a double-edged sword, as excessive ROS can oxidize proteins, damage the DNA structure, and induce cell apoptosis. Moreover, ROS can induce inflammation at the tumor site, which further improves tumor immunogenicity. Therefore, increasing the content of ROS in tumor sites has become an effective method for cancer therapy. At present, the ways to generate ROS through external stimulations, such as photodynamic reaction, sonodynamic reaction, and radiation sensitization, are greatly limited by the penetration depth of the laser, irradiation range of external excitation, and safety concerns of the radiation.
In response to these problems, chemodynamic therapy has been developed, which has received widespread attention. Chemodynamic therapy uses excess H2O2 in the tumor microenvironment without external energy stimulation to generate ROS through the Fenton reaction. However, the current therapeutic effect of chemodynamic therapy is not satisfactory, because the initiation of an efficient Fenton reaction requires harsh acidic conditions and excess H2O2. In addition to exogenous ROS production strategies, increasing the generation of endogenous ROS to inhibit tumor growth is another promising approach. It is well known that inhibiting the mitochondrial electron transport chain can enhance the generation of ROS. However, treating cancer only by increasing endogenous ROS is unsatisfactory, as it is difficult to effectively inhibit tumor growth with a low amount of produced endogenous ROS. Therefore, developing strategies for the selective generation of sufficient ROS without external energy stimulation under mild in vivo conditions remains a huge challenge in the field of cancer therapy.
Source: Read Full Article