IntroductionChronopathologyChronotherapeuticsConclusionReferencesFurther reading Chronobiology is an upcoming field of study that leaves many bewildered as to its scope. This branch of
Read moreWellness
Health news, stories and tips that inspire healthy diets, relationships and lives …
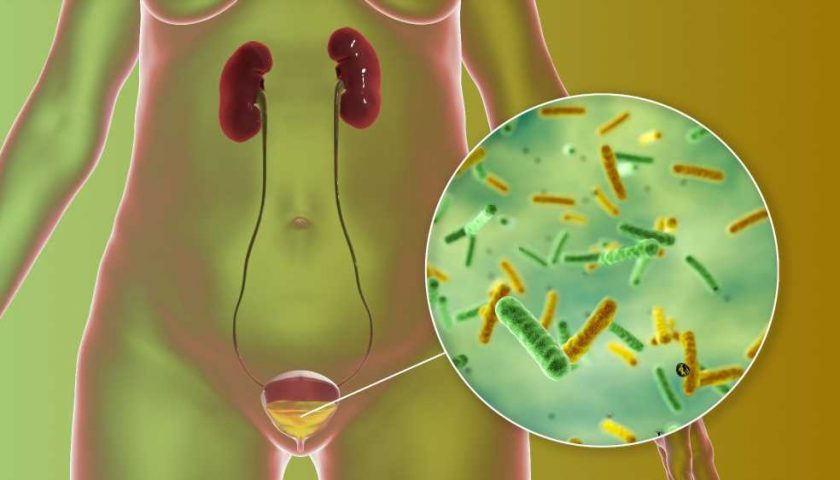
The Urobiome: Unveiling its Role in Human Health and Disease
The urinary microbiomeOriginUrobiome in UTIsThe urobiome in the sexesThe urobiome with ageRenal disease and the urobiomeThe urobiome and cancerThe urobiome
Read moreTau-based biomarker tracks Alzheimer’s progression
Two pathologies drive the progression of Alzheimer’s disease. Early on, amyloid beta plaques lead the way, but around the time
Read moreTransforming Vaccine Design: The Promise of Systems Biology
The current state of vaccine designWhat is systems biology?The impact of systems biology on vaccine designWhat challenges are involved?What does
Read moreExblifep
Generic name: cefepime and enmetazobactam Treatment for: Urinary Tract Infection Allecra Therapeutics Submits New Drug Application to the U.S. FDA
Read moreAntibiotic Resistance and Urinary Tract Infections
The problem of resistance The invadersResistance to common drugs Current options ReferencesFurther reading Urinary tract infections (UTIs) are among the most common infections
Read moreCurrent Challenges Surrounding Clostridium Difficile Infection
Clostridium difficile: a highly successful human gut pathogenChallenges in managing clostridium difficile infectionC. difficile as a ubiquitous microorganismFaecal microbiota transplant
Read moreHow are Drones Used in Healthcare?
How could drones revolutionize healthcare?Drone usage in life-threatening conditionsZipline: drone delivery of medical suppliesChallengesReferencesFurther reading Drones in healthcare are gaining
Read moreManaging Emotions during the COVID-19 Struggle
The stress of modern-day life has given birth to fluctuating feelings in most of us. So many factors influence our
Read moreGordon wins Spain’s Asturias Award
Microbiome pioneer Jeffrey I. Gordon, MD, of Washington University School of Medicine in St. Louis, has won the 2023 Princess
Read more