In a recent study published on the medRxiv* preprint server, researchers report an overview of antibody neutralization by all currently approved vaccines against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron, Beta, and Delta variants of concern (VOCs) in the European Union and the United Kingdom.
Study: Diminished neutralization responses towards SARS-CoV-2 Omicron VoC after mRNA or vector-based COVID-19 vaccinations. Image Credit: Viacheslav Lopatin / Shutterstock.com
An unusually high number of amino acid alterations in the Omicron spike (S) protein has raised concerns that this VOC will likely pose an increased reinfection risk or breakthrough infections in vaccinated individuals. While several studies have shown loss of neutralization capacity against Omicron via in vitro binding assays, data on antibody responses against Omicron and other VOCs in various vaccination regimens over time is sparsely available.
About the study
In the current study, researchers collected serum samples from vaccinated participants of a population-based SARS-CoV-2 seroprevalence study held in Germany from July 2020 to August 2021. These individuals had either received a single dose of Ad26.COV2.S, homologous two-doses of BNT162b2, mRNA-1273, or AZD1222 vaccines, or heterologous two-doses of AZD1222-BNT162b2 or AZD1222-mRNA-1273 vaccines.
The researchers analyzed sera by in vitro pseudotype particle neutralization assay. They recorded the neutralization responses towards the S protein of SARS-CoV-2 B.1, Omicron (B.1.1.529), Beta (B.1.351), and Delta (B.1.617.2) variants approximately four weeks after vaccination. Neutralization responses were also recorded after six months for longitudinal follow-up of BNT162b2 vaccine recipients.
Study findings
The parental wild-type SARS-CoV-2 strain (B.1) showed a response rate of 73% against Ad26.CoV2.S vaccine. However, when assessed against the Beta and Omicron variants, response rates significantly reduced to 18% and 9%, respectively.
Homologous vaccination with either AZD1222 or BNT162b2 performed better against the Omicron variant with 50% and 33% responders, respectively. The response rate of heterologous immunization with AZD1222-BNT162b2 and AZD1222-mRNA-1273 were 80% and 82%, respectively. Notably, homologous immunization with mRNA-1273 was most effective against the Omicron variant, with a response rate of 100%.

Impact of SARS-CoV-2 vaccination schemes on neutralization response towards Omicron (B.1.1.529) variant.
The SARS-CoV-2 Omicron variant showed the highest neutralization titers across all the tested samples. The World Health Organization (WHO) international standard control serum showed detectable neutralization against all variants, including Omicron, showing excellent sensitivity towards the assay used in this study.
Since BNT162b2 is the most commonly used vaccine around the world, the researchers did a follow-up study on BNT162b2 vaccine recipients six months post-vaccination. During the follow-up period, BNT162b2 recipients showed a response rate of 47% against the Omicron variant. However, response rates against Omicron remained well-maintained over time, thus indicating sustained cross-protection against this variant.
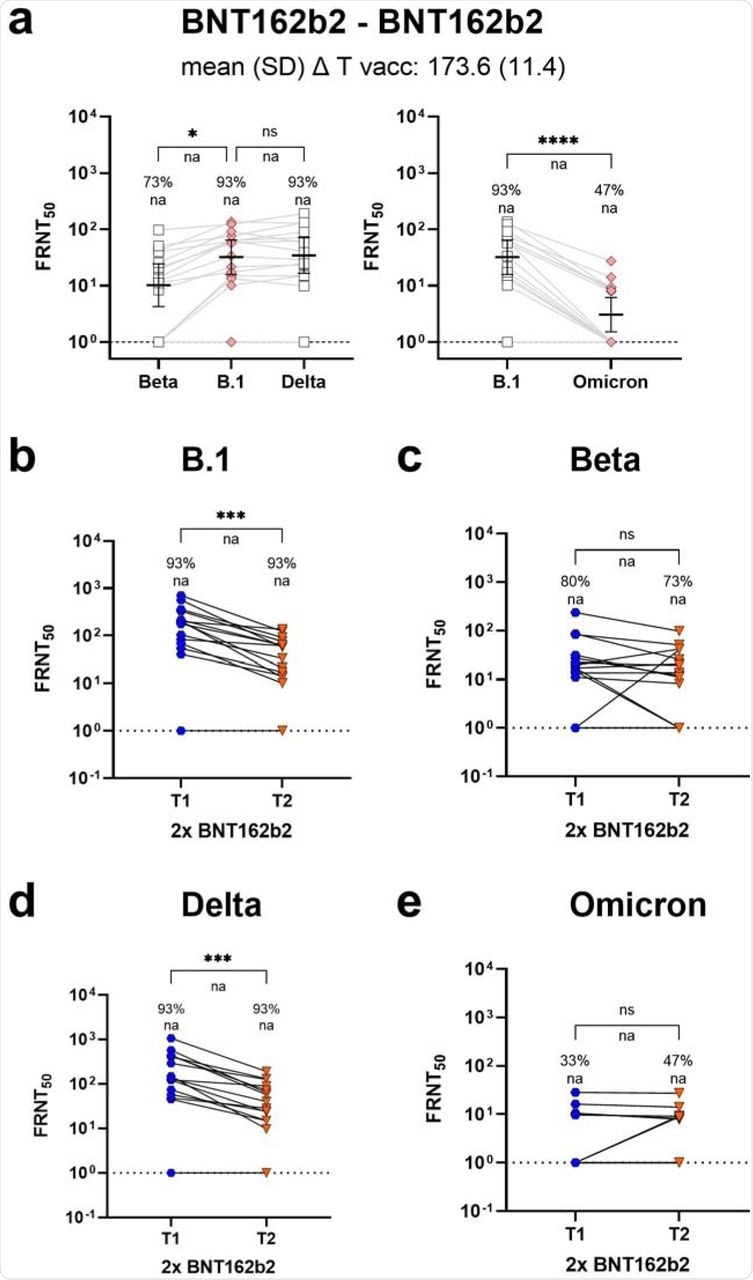
Longitudinal BNT162b2 neutralization response towards SARS-CoV-2 VoC Omicron (B.1.1.529).
For an in-depth assessment of the immune-evading capabilities of these VOCs, the researchers compared geometric mean titers (GMT) and focus reduction neutralization titer with a 50% neutralization cut-off (FRNT50) values; however, they excluded GMT fold-changes for groups that included non-responders. Non-responders were study participants with undetectable neutralization titers at an initial serum dilution of 1:10.
For each vaccination regimen, the researchers calculated fold changes separately on a subset of samples that showed detectable titers under all circumstances. Subsequently, an approximately 15-fold reduction in GMT values for most vaccinations was observed, except for BNT162b2, where the reduction was 28-fold during peak responses. This finding complemented the high frequency of non-responders, which suggested weaker protection against the Omicron VOC.
Paired sera from BNT162b2 vaccine recipients at four weeks and six months after the second dose allowed researchers to compare intra-individual titer changes over time. While the neutralization of the SARS-CoV-2 B.1 and Delta strains decreased significantly over time, the time-dependent reduction was less pronounced for the Beta and Omicron VOCs.
Moreover, all Omicron responders identified soon after vaccination had detectable neutralizing capacity at a later time point. Therefore, the differences in neutralization titers between B.1 and Omicron responders were less pronounced at late time points than at the peak.
Conclusions
To summarize, the study findings suggest that homologous mRNA-1273 vaccination resulted in the highest response rate, whereas the lowest response was observed in individuals who received the Ad26.CoV2.S vaccine. All mRNA-1273 vaccine recipients and 80% of heterologous vaccine recipients showed detectable neutralization against the Omicron variant.
The neutralization potential of vector-based vaccines was poor towards the Omicron variant, even during the peak phase, shortly after vaccination. However, if Omicron responses occurred at all, although less, they were durable, as suggested by the findings of the longitudinal six-month follow-up. Furthermore, these follow-up studies revealed that BNT162b2 recipients showed very low cross-neutralization.
The current study provides accumulating evidence that mutations in the Omicron S protein help it evade vaccine-induced protection. Booster vaccination is advised before six months, especially for coronavirus disease 2019 (COVID-19) risk groups, considering the unavailability of a precise and clinically relevant correlate of protection.
*Important notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Jacobsen, H., Strengert, M., Maa, H., et al. (2021). Diminished neutralization responses towards SARS-CoV-2 Omicron VoC after mRNA or vector-based COVID-19 vaccinations. medRxiv. doi:10.1101/2021.12.21.21267898. https://www.medrxiv.org/content/10.1101/2021.12.21.21267898v1.
Posted in: Medical Research News | Disease/Infection News | Pharmaceutical News
Tags: Amino Acid, Antibody, Assay, Coronavirus, Coronavirus Disease COVID-19, Frequency, Homologous, Immunization, in vitro, Protein, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Vaccine

Written by
Neha Mathur
Neha Mathur has a Master’s degree in Biotechnology and extensive experience in digital marketing. She is passionate about reading and music. When she is not working, Neha likes to cook and travel.
Source: Read Full Article