Omicron variant causes fear as it continues to spread rapidly
Fox News correspondent Jonathan Serrie reports the latest on ‘Special Report’ as the virus spreads faster than previous variants.
More than 800,000 Americans have died since the beginning of the COVID-19 pandemic.
The introduction of vaccines at the end of 2020 and the beginning of 2021 brought hope for many — even as cases spiked at the end of the summer, largely due to the delta variant and the unvaccinated.
Meanwhile, vaccine-makers have worked to meet demand and respond to additional concerns, like the recently identified omicron variant.
Numerous breakthroughs in scientific achievement led to where the U.S. and the rest of the world stand today — though the pandemic’s future remains uncertain, evista medication side effects especially as cases spike this winter.
Here are a few of the most important milestones:
Feb. 27 — The FDA grants EUA to Johnson & Johnson’s COVID-19 vaccine
On Feb. 27, the U.S. Food and Drug Administration (FDA) authorized the Johnson & Johnson Janssen COVID-19 vaccine for emergency use.
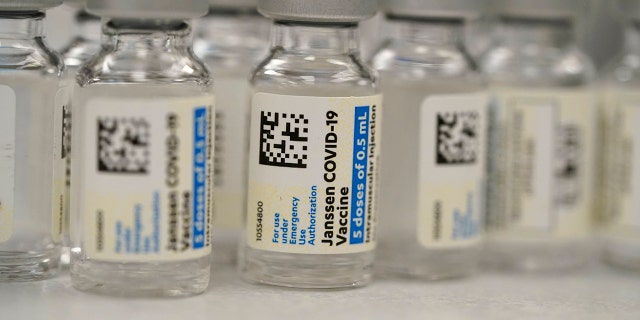
This Saturday, March 6, 2021, file photo shows vials of the Johnson & Johnson Janssen COVID-19 vaccine in the pharmacy of National Jewish Hospital for distribution in east Denver.
(AP Photo/David Zalubowski, File)
Unlike the Moderna and Pfizer-BioNTech two-dose primary series, the Johnson & Johnson vaccine takes just one shot.
Aug. 12 — The FDA authorizes additional vaccine dose for immunocompromised
At the time, the vaccine was also authorized for emergency use for individuals 12-15 years of age.
“The FDA’s approval of this vaccine is a milestone as we continue to battle the COVID-19 pandemic,” acting FDA Commissioner Janet Woodcock said in a statement. “While this and other vaccines have met the FDA’s rigorous, scientific standards for emergency use authorization, as the first FDA-approved COVID-19 vaccine, the public can be very confident that this vaccine meets the high standards for safety, effectiveness, and manufacturing quality the FDA requires of an approved product.”
Nov. 2 — CDC director endorses recommendation to vaccinate ages 5-11
On Nov. 2, Centers for Disease Control and Prevention (CDC) Director Dr. Rochelle Walenksy signed off on distributing the Pfizer-BioNTech COVID-19 vaccine to children ages 5-11.
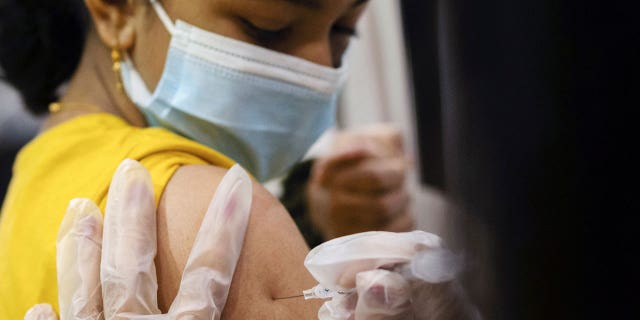
A girl receives the Pfizer-BioNTech coronavirus disease (COVID-19) vaccine in Lansdale, Pennsylvania, Dec. 5, 2021.
(REUTERS/Hannah Beier)
The FDA had authorized emergency use for kid doses — which are about one-third of the dose given to adolescents and adults — a week before her endorsement.
Nov. 19 — The FDA authorizes Pfizer-BioNTech and Moderna COVID-19 vaccines AND boosters for all adults
The CDC also recommended the use of boosters for all adults on the same day.
Nov. 26 — WHO designates omicron ‘variant of concern’
Just a week later, the World Health Organization (WHO) classified B.1.1.529, or the omicron variant, as a “variant of concern.”
This variant has numerous mutations, and scientists are working to determine its severity, transmissibility and ability to evade immune protection and vaccines.
Omicron has been detected in 36 states and more than 75 countries worldwide.
Source: Read Full Article