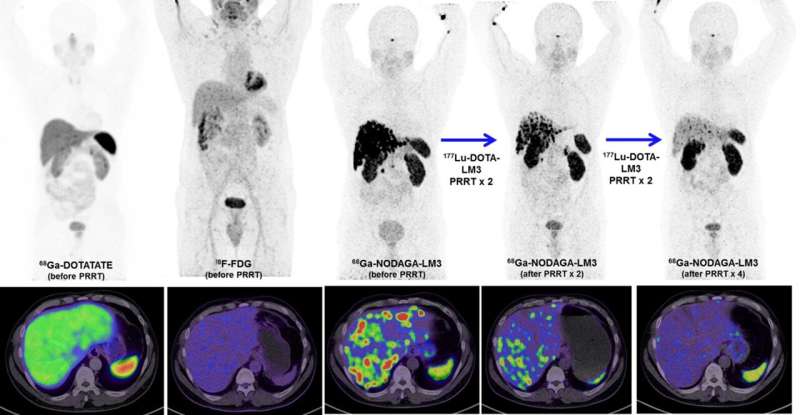
A new type of peptide receptor radionuclide therapy (PRRT) has been shown to control disease in 85 percent of patients with metastatic neuroendocrine neoplasms, achieving complete remission in some patients. The first-in-human study utilized 177Lu-DOTA-LM3 therapy, which was administered without severe adverse effects and was well tolerated by the majority of patients. This research was published in the November issue of The Journal of Nuclear Medicine.
Neuroendocrine neoplasms are tumors that arise from diffuse neuroendocrine system cells. They are most commonly found in the gastrointestinal tract, pancreas and lungs. Neuroendocrine neoplasms are fairly rare diseases, but their incidence and prevalence have increased substantially in recent decades. The majority of neuroendocrine tumors overexpress somatostatin receptors (SSTRs), which are targeted for imaging and treatment.
“Over the past two decades, SSTR-targeted imaging using radiolabeled somatostatin agonists followed by PRRT has been remarkably successful in managing neuroendocrine tumors,” said Jingjing Zhang, MD, Ph.D., assistant professor in the Department of Diagnostic Radiology at the Yong Loo Lin School of Medicine at the National University of Singapore in Singapore. “However, potent SSTR antagonists—which only poorly internalize into tumor cells if at all—have surprisingly been shown to be even superior to agonists for such purposes.”
To further investigate the role antagonists can play in treating neuroendocrine tumors researchers developed a study to determine the safety, biodistribution and efficiency of a new type of SSTR antagonist, 177Lu-DOTA-LM3. Fifty-one patients with progressive, heavily pretreated neuroendocrine neoplasms underwent PRRT with 177Lu-DOTA-LM3. Treatment-related adverse events were graded for all participants, and dosimetry was performed for 11 patients.
177Lu-DOTA-LM3 was administered without severe adverse effects and was well tolerated by most patients. Disease control was reached in 40 out of 47 patients (85 percent) who were monitored after 177Lu-DOTA-LM3 therapy. Two patients achieved complete remission by the European Organization for Research and Treatment of Cancer criteria.
Of note, the uptake and dosimetry of the antagonist 177Lu-DOTA-LM3 were compared with those of the commonly used SSTR agonist 177Lu-DOTATOC in patients undergoing treatment on the same dosimetry protocol. 177Lu-DOTA-LM3 demonstrated higher uptake and a longer effective half-life in tumor lesions, resulting in higher tumor radiation doses than for agonist 177Lu-DOTATOC.
Source: Read Full Article