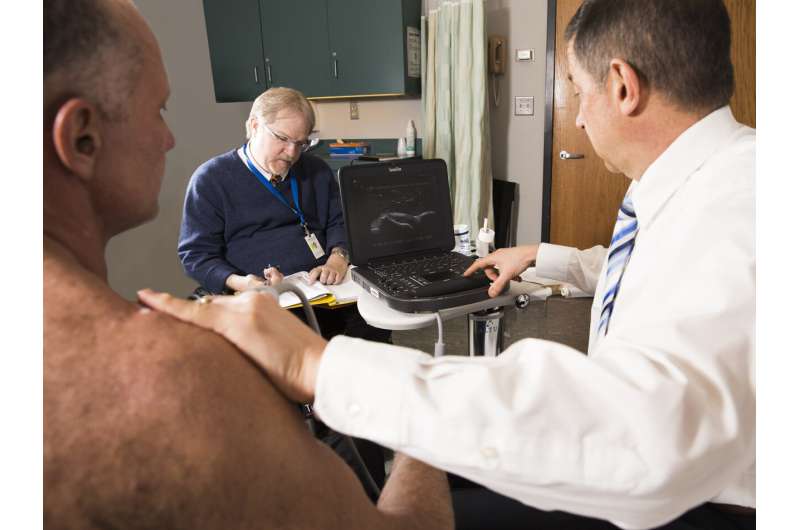
A team of specialists in regenerative rehabilitation conducted a successful pilot study investigating micro-fragmented adipose tissue (MFAT) injection for rotator cuff disease in wheelchair users with spinal cord injury. They demonstrated that MFAT injection has lasting pain-relief effects. The article, “A pilot study to evaluate micro-fragmented adipose tissue injection under ultrasound guidance for the treatment of refractory rotator cuff disease in wheelchair users with spinal cord injury,” was published ahead of print on April 8, 2021, by the Journal of Spinal Cord Medicine.
The authors are Trevor Dyson-Hudson, MD, and Nathan Hogaboom, Ph.D., at Kessler Foundation; and Gerard Malanga, MD, a founder of the New Jersey Regenerative Institute and visiting scientist at Kessler Foundation, and Chris Cherian, MD, of Rutgers New Jersey Medical School. The study was conducted at the Derfner-Lieberman Laboratory for Regenerative Rehabilitation Research in the Center for Spinal Cord Injury Research at Kessler Foundation.
Shoulder pain is a common occurrence among wheelchair users with spinal cord injury because they rely solely on their upper limbs to perform everyday tasks. Often, pain is caused by soft-tissue injuries such as damage to rotator cuff tendons. Many non-surgical therapies for shoulder pain exist, including pain medication, physical therapy, and equipment modifications, but these have shown limited efficacy. Persistent shoulder pain can significantly lessen quality of life, and if conservative therapies fail, shoulder surgery is frequently the only option, which comes with its own set of risks and potential setbacks.

In this single-group pilot study, researchers explored the efficacy of a minimally invasive biological intervention involving an ultrasound-guided injection of MFAT, which harbors a potential source of bioactive and regenerative components for orthopedic conditions and may provide cushioning that can improve function and alleviate pain caused by rotator cuff injuries.
Ten wheelchair users with chronic spinal cord injury who had moderate-to-severe shoulder pain for more than six months caused by refractory rotator cuff disease participated in the study. All received an injection of MFAT and were evaluated at six and 12 months after treatment. Evaluation metrics included the 11-point Numerical Rating Scale, the Wheelchair User’s Shoulder Pain Index, Brief Pain Inventory pain interference items (BPI-17), Patient Global Impression of Change, ultrasound and physical examinations, and adverse events.
The results were encouraging, according to Drs. Hogaboom and Dyson-Hudson, co-directors of the Derfner-Lieberman Laboratory. Nearly 80 percent saw a meaningful decrease in pain symptoms, and all but one reported some improvement in pain and function. Moreover, scores declined steadily over the first three months for all metrics, and over the entire year for the BPI-17 metric, suggesting that this intervention has long-lasting effects. There were no significant adverse events.
Source: Read Full Article