A recent study currently under review at Scientific Reports journal and posted to the Research Square* preprint server demonstrated a potential positive correlation between reactogenicity and immunogenicity of coronavirus disease 2019 (COVID-19) vaccination.
 Study: Predictive value of reactogenicity for anti-SARS-CoV-2 antibody response in mRNA-1273 recipients: a multicenter prospective cohort study. Image Credit: NIAID
Study: Predictive value of reactogenicity for anti-SARS-CoV-2 antibody response in mRNA-1273 recipients: a multicenter prospective cohort study. Image Credit: NIAID
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) messenger ribonucleic acid (mRNA) vaccination has been introduced to combat the COVID-19 pandemic. The national SARS-CoV-2 vaccination campaign in South Korea has been steadily broadened to incorporate the COVID-19 mRNA-1273 vaccine, which is the fourth SARS-CoV-2 vaccine approved as of May 2021. mRNA-1273 is a SARS-CoV-2 mRNA lipid nanoparticle vaccine that induces an antibody response to the SARS-CoV-2 spike (S) protein in real-world studies and clinical trials. Nevertheless, there is limited information on antibody kinetics and variables that influence the immunogenicity of the mRNA-1273 vaccine, especially in Asian nations.
mRNA-1273 was more immunogenic and highly associated with adverse events (AEs) than the SARS-CoV-2 BNT162b2 vaccine in clinical trials. Furthermore, certain groups of people vaccinated with mRNA-1273, such as the younger population, had a robust immunological response with more AEs. These synchronous correlations might support the concept that greater reactogenicity is linked to better immunogenicity. Previous studies have documented this link, although the findings have varied depending on the vaccine type.
About the study
In the current prospective research, the investigators assessed the immunogenicity of the COVID-19 mRNA-1273 vaccine and its association with AEs in young, healthy adults to address the existing uncertainties. The study was performed at four University hospitals (Korea University Guro Hospital, International St. Mary’s Hospital, Ajou University Hospital, and Kangnam Sacred Hallym University Hospital) in June 2021 among healthy young individuals aged 19 to 55 years willing to be immunized with the mRNA-1273 vaccine.
All participants submitted written informed consent before enrolling in the study. In addition, data on demographics and comorbidities were obtained from each subject. People excluded from the investigation were those with prior laboratory-validated SARS-CoV-2 infection, a history of autoimmune disease, pregnant, breastfeeding, or immunocompromised.
The subjects were vaccinated twice with the mRNA-1273 vaccine over a 28-day gap. The authors procured blood samples from the volunteers and prospectively examined adverse reactions following the administration of each vaccine dose.
Further, the team assessed the association between vaccine reactogenicity and humoral immune response. The anti-S antibody was quantified to determine immunogenicity. Participants were asked to complete a validated electronic questionnaire a week after receiving each vaccine dose to document the severity, duration, and incidence of solicited AEs. Additionally, data on antipyretics usage were obtained following each vaccination dose receipt.
Results and discussions
The study results depicted transient humoral immune response in mRNA-1273-vaccinated young, healthy adults around eight weeks post-vaccination. The authors found that following the initial and second vaccine doses, 177 subjects aged between 19 and 55 years had an anti-SARS-CoV-2 S immunoglobulin G (IgG) antibody geometric mean titers (GMTs) of 178.07 U/mL and 4409.61 U/mL, respectively. Furthermore, their 50% neutralizing (ND50) titers four weeks following the first and second vaccine shots were 479.95 U/mL and 2851.67 U/mL, respectively.
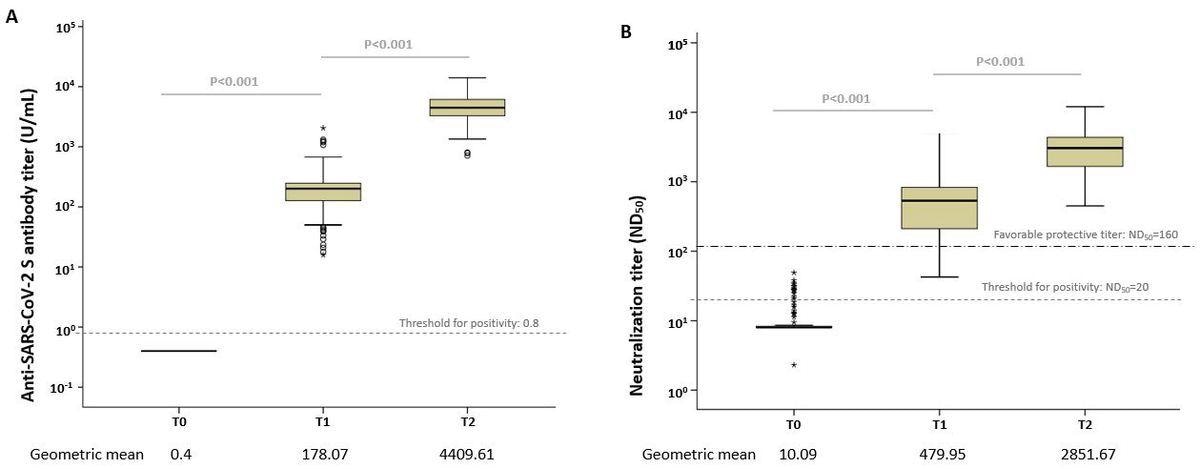
Box plots of the anti-SARS-CoV-2 antibody levels. (A) Anti-S IgG antibody and (B) median neutralizing titer (ND50) at each time point. The dotted line shows the threshold for positivity. Open circles depict outliers.
The neutralizing antibody concentrations achieved a titer range considered positive, i.e., ≥160, based on the Food and Drug Administration (FDA) standards in 85% of subjects following only one dose and 100% of volunteers after two-dose vaccination. This finding revealed that the mRNA-1273 vaccination elicited a significant immune response in young adults.
Anti-S IgG antibody levels induced by both doses were not linked to local reactogenicity. Yet, anti-S IgGs were elevated considerably in those who suffered systemic adverse effects such as fever, muscle pain, or headache. Systemic AEs were more common following the second dose versus after the first; over 70% of the subjects reported chills, fever, headache, fatigue, and muscle pain following the vaccination with the second dose. Further, antipyretic usage was an autonomous predictor of high anti-SARS-CoV-2 antibody response following both doses.
In addition, systemic reactogenicity following the initial vaccine shot impacted antibody response post-second dose vaccination. The authors postulated that antigenic priming of the immune system following the initial vaccine dose could result in enhanced reactogenicity upon the successive antigenic exposure. Based on this assumption, they hypothesized that heightened reactogenicity following mRNA-1273 vaccination, specifically systemic AEs post-second dose vaccination, was strongly linked to higher immunogenicity.
Conclusions
Overall, the study findings showed that the SARS-CoV-2 mRNA-1273 vaccination elicited a strong antibody response in healthy young people. The scientists mentioned that systemic reactogenicity and post-vaccination immunogenicity might be linked. Interestingly, they found that after mRNA-1273 immunization, antipyretic usage did not affect the anti-SARS-CoV-2 antibody response. The findings indicated that antipyretics use was an objective marker of systemic reactogenicity following vaccination.
*Important notice
Preprints with Research Square publish preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Min Joo Choi, Jung Yeon Heo, Yu Bin Seo et al. Predictive value of reactogenicity for anti-SARS-CoV-2 antibody response in mRNA-1273 recipients: a multicenter prospective cohort study, 23 May 2022, PREPRINT (Version 1) available at Research Square https://doi.org/10.21203/rs.3.rs-1622167/v1, https://www.researchsquare.com/article/rs-1622167/v1
Posted in: Medical Research News | Disease/Infection News | Pharmaceutical News
Tags: Antibody, Autoimmune Disease, Blood, Breastfeeding, Coronavirus, Coronavirus Disease COVID-19, covid-19, Fatigue, Fever, Food, Headache, Hospital, Immune Response, Immune System, Immunization, Immunoglobulin, Laboratory, Muscle, Nanoparticle, Pain, Pandemic, Protein, Research, Respiratory, Ribonucleic Acid, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Vaccine

Written by
Shanet Susan Alex
Shanet Susan Alex, a medical writer, based in Kerala, India, is a Doctor of Pharmacy graduate from Kerala University of Health Sciences. Her academic background is in clinical pharmacy and research, and she is passionate about medical writing. Shanet has published papers in the International Journal of Medical Science and Current Research (IJMSCR), the International Journal of Pharmacy (IJP), and the International Journal of Medical Science and Applied Research (IJMSAR). Apart from work, she enjoys listening to music and watching movies.
Source: Read Full Article